Abstract
Vitamin A levels in fattening Japanese Black cattle affect meat quality; therefore, it is important to monitor serum retinol concentrations. To simplify and accelerate the evaluation of serum retinol concentrations in cattle, we developed a new predictive method using excitation-emission matrix (EEM) fluorescence spectrophotometry. For analytical comparison, the concentration of serum retinol was also measured using the conventional HPLC method. We examined excitation (Ex) and emission (Em) wavelengths of cattle serum, which were 250–450 and 250–600 nm, respectively. Parallel factor analysis separated four components from EEM data, one of which was related to retinol. Next, a partial least square regression model was created using the obtained EEMs as explanatory variables and accrual measurement values as objective variables. The determination coefficient value (R2), root mean squared error of prediction (RMSEP), and the ratio of performance to deviation (RPD) of the model were determined. A comparison with reference values found that R2, RMSEP, and RPD of the calibration model were 0.95, 6.4 IU/dl, and 4.2, respectively. This implies that EEM can estimate the serum retinol concentration with high accuracy. Additionally, the fluorescent peaks that contributed to the calibration, which were extracted from the regression coefficient and variable importance in projection plots, were Ex/Em = 320/390 and 330/520 nm. Thus, we assume that this method observes not only free retinol, but also retinol-binding protein. In conclusion, multidimensional fluorescence analysis can accurately and quickly determine serum retinol concentrations in fattening cattle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin A is often restricted in the feed of fattening Japanese Black cattle. This improves beef meat quality, increasing marbling, decreasing subcutaneous fat, and increasing the loin eye area [1,2,3]. However, excessive vitamin A restriction can induce hypovitaminosis A, which results in growth stunting, low immunity, and night blindness [4, 5]. Therefore, vitamin A in fattening Japanese Black cattle needs to be carefully monitored.
Vitamin A deficiency can be detected by measuring the serum retinol concentration. This is commonly done by taking an animal blood sample, pretreating it with an organic solvent, and analyzing it using high performance liquid chromatography (HPLC) [6]. However, HPLC is time intensive and has a high running cost. An alternative method that is simple and quick is desirable. Direct fluorescence has been used to determine serum retinol concentrations in human blood [7, 8]. However, the method is complex, requiring a hundred-fold dilution in 0.1 M NaCl solution. Hence, we propose that simplification of sample preparation will lead to high-throughput monitoring of the serum retinol concentration.
Recently, multidimensional fluorescence has been used to detect metabolites in biological samples to evaluate the quality of various animal products [9,10,11,12]. Multidimensional fluorescence is comprehensive, has a high sensitivity, and requires minimal sample preparation steps. It has been used to directly measure biological samples and agricultural products that consist of many compounds. Front-face fluorescence spectroscopy coupled with multivariate analysis has been used to measure β-carotene, tryptophan, vitamin A (retinol), and riboflavin concentrations in milk and dairy products [13,14,15,16]. Multidimensional fluorescence has also been used with human blood, to quantify riboflavin, aromatic amino acids, and coenzymes (i.e. NADH and FAD) [17,18,19]. Therefore, this method could potentially be applied to estimate the retinol concentration in cow serum. The aim of this study was to develop a simple high-throughput monitoring tool for serum retinol using multidimensional fluorescence. This will help monitor the health status of fattening Japanese Black cattle.
Materials and Methods
Cattle Serum Samples
This study was conducted from June to October, 2019, using fattening Japanese Black cattle bred at Yuzukami farm (Tochigi, Japan), according to “standards relating to the care and keeping of industrial animals” by the Ministry of the Environment, Government of Japan. Blood samples were collected from the jugular veins of 171 castrated males and 37 females (approximately 8–27 months old; Table 1), using VENOJECT II needles (Terumo medical Corp., Tokyo, Japan) and 8-ml serum separator vacuum tubes (Kyokuto Pharmaceutical Industrial Co., Ltd., Tokyo, Japan). The separating tubes were immediately centrifuged (2000×g, 4 °C, 30 min) to separate the upper phase. Serum samples were then stored at −80 °C until further analysis.
HPLC Quantitation of Retinol
Retinol quantitation was conducted at Kotobiken medical laboratories, Inc. (Ibaraki, Japan). Retinol was extracted using 400 μl of methanol and 400 μl of the serum sample. The mixture was centrifuged, and the supernatant was then measured by an HPLC/RF-20Axs (Shimadzu Corp., Kyoto, Japan).
Excitation-Emission Matrix (EEM) Fluorescence Spectrophotometry
To obtain excitation-emission matrices (EEMs) of serum samples, we used a fluorescence spectrophotometer via a micro-plate reader (F-7100; Hitachi high-tech science Corp., Tokyo, Japan). This instrument was equipped with an automatic filtering attachment to remove multi-order light. The conditions used were as follows: photomultiplier voltage, 375 V; response time, 2 ms; scanning speed, 30,000 nm min−1; excitation (Ex) wavelength range, 250–450 nm; emission (Em) wavelength range, 250–600 nm; Ex wavelength intervals, 5 nm; and Em wavelength intervals, 1 nm. The plates used were Nunc F96 MicroWell 96-well black polystyrene plates (Thermo Fisher Scientific Inc., MA, United States). Serum samples were pipetted into the micro-well plates (300 μl/well) without pretreatment. Front-face fluorescence spectroscopy was used with three replicates for each sample, performed at 3 min/scan. Data were pre-processed to eliminate unnecessary effects of Raman scattering and multi-order light from the EEM using software FL solution 4.2 (Hitachi high-tech science Corp., Tokyo, Japan). Processed EEMs were outputted as a two-way data matrix for a partial least square (PLS) regression.
Statistical Analysis
To determine significant wavelength ranges of different retinol concentration levels in cattle serum, R packages EEM (https://cran.rstudio.com/web/packages/EEM/index.html) and stardom (https://cran.r-project.org/web/packages/staRdom/index.html) were used. The EEM package was used to make contour plots of non-processed raw data. The stardom package was used to separate components of the EEMs with parallel factor analysis (PARAFAC). PARAFAC was conducted for the three-way data array following an excitation × emission × analytical sample of 41 × 351 × 624. Moreover, Pearson’s correlation was calculated with the component score value and reference value to verify whether there is a quantitative relationship between retinol concentrations and their fluorescence intensities. To create a calibration model of serum retinol concentration and determine the prediction ability of this model, all EEMs were randomly divided into calibration (n = 145) and validation (n = 63) sample sets with three analytical replicates for each sample. The calibration model was built with normalized EEM data (X) and reference values (Y) using PLS regression on SIMCA 15 software (Umetrics, Sweden). The EEM data were normalized using unit variance scaling. The evaluation indices used were coefficient of determination (R2), root mean square error of evaluation (RMSEE), and root mean square error of prediction (RMSEP). The calibration model had a high R2 and low RMSEE and RMSEP values, indicating high accuracy. Additionally, the ratio of standard deviation of reference data in the validation set to RMSEP (RPD) was calculated to evaluate the calibration model, where RPD < 1.5 indicates preliminary screening, 2.0–2.5 indicates satisfactory prediction, and > 2.5 indicates good prediction [20,21,22]. The regression coefficients of the calibration model were indexed to determine which Ex/Em wavelengths contributed positively or negatively to the regression model of the target variable. Moreover, the variable of importance projection (VIP) values greater than 1 were regarded as important to the estimation of the response variable [9, 22]. The workflow of this study is shown in Fig. 1.
Results and Discussion
Excitation- and Emission-Matrix Characteristics of Cattle Serum
The HPLC quantitation results of retinol based on calibration and validation sample sets are shown in Table 1. To investigate EEM characteristics associated with retinol and retinol-related compounds, raw EEMs of cattle serum containing high (124.0 IU/dl) and low (11.0 IU/dl) retinol concentrations were contrasted (Fig. 2). Contour plots of these samples showed common peaks at Ex/Em 290/340 nm and 270/340 nm. Two observed peaks almost corresponded with wavelength ranges of tryptophan residues (Ex/Em, 290/300–400 nm) [23]. However, a peak related to retinol could not be confirmed (Fig. 2). Therefore, we conducted PARAFAC to statistically extract EEM characteristics. Previously, PARAFAC analysis has successfully detected fluorophores such as tryptophan, vitamin B6, and riboflavin from EEMs of brined of salted herring [24]. In the present study, PARAFAC modeling extracted four components with maximum excitation and emission loadings of 285/342 nm, 300/350 nm, 270/320 nm, and 325/447 nm (Fig. 3a). However, an Ex/Em of 280–290/340–370 nm has been found to be related to aromatic amino acids such as tryptophan and tyrosine in dairy and meat products [11, 13]. Therefore, we presumed that the first three components we extracted were related to aromatic amino acids in cattle serum. Conversely, retinol in cow-derived milk and cheese samples have been found to emit at 410, 411 and 435 nm when excited at 320 nm using front-face fluorescence [13, 15]. Vitamin-A (retinol) associated with the quality of buffalo milk and camel milk has been detected with a fluorescence emission at 410 nm and 442 nm (excitation at 320 nm and 322 nm), respectively [25, 26]. Considering the information of retinol fluorescence in the previous studies and our experimental results, the fourth component extracted using PARAFAC (325/447 nm) was assigned to retinol in cattle serum. Moreover, we found a significant correlation (P < 0.001) between score values derived from the fourth component and retinol concentrations measured using HPLC (Fig. 3b). This confirms that EEM characteristics can be used to predict serum retinol concentrations in fattening cattle.
Prediction of Serum Retinol Concentration Using Multidimensional Fluorescence
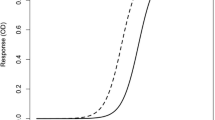
We created a calibration model to predict the serum retinol concentration. Analysis of the model found that it had good accuracy (RMSEE = 5.6, R2 = 0.95; Fig. 4a) and was robust [intercepts from permutation test were: R2 = (0.000, 0.005), Q2 = (0.000, −0.147)] [21, 27]. Next, we evaluated the predictability of the calibration model using the validation sample set. Evaluation indices were as follows: RMSEP = 6.3 IU/dl; R2 = 0.95; RPD = 4.2 (Fig. 4b). The RPD value exceeded 2.5, indicating that the calibration model can predict serum retinol concentrations with high accuracy.
Relationship between reference values and predicted values using a partial least square (PLS) regression model using a the calibration data set and b the validation data set, where RMSEE is the root mean squared error of evaluation, RMSEP is the root mean squared error of prediction; and RPD is the ratio of performance to deviation
Fluorescence Contributions from Serum Retinol in Fattening Cattle
We determined the contribution of Ex and Em wavelengths to serum retinol concentration prediction. Contour plots of the PLS regression coefficients and the VIP values are shown in Fig. 5. Importance variables of the calibration model are usually regarded as VIP >1. The main peaks of the VIP contour plot were at 320/390 nm and 330/530 nm (Fig. 5b), with negative and positive regression coefficients, respectively (Fig. 5a). The peak of 320/390 nm roughly corresponded with the Ex/Em wavelength of retinol [15]. A direct fluorescent measurement of retinol concentrations in human serum diluted with 0.1 M NaCl used a 335 nm excitation and 460 nm emission peak [7, 8]. Furthermore, Watanabe et al. (2008) [28] suggested the measurement of serum retinol using a fluorescence microplate reader with an Ex/Em of 335/510 nm. However, their method targeted retinol-binding protein, which has a higher fluorescence intensity than free retinol. Therefore, in our study, both free and bound retinol might contribute to Ex/Em peaks.
For the first time, we have succeeded in applying multidimensional fluorescence to the estimation of retinol content in cattle serum. Our new method is able to make monitoring vitamin A in fattening Japanese Black cattle easier and cheaper, without cumbersome operations and reagents. However, it might be difficult to apply our regression model to samples from cattle under different conditions that can affect the composition of blood metabolites. For example, heat-stress can affect plasma metabolite profiles (e.g. concentrations of carbohydrates, lipids, and amino acids) in cattle [27]. Additionally, the composition of blood metabolites in cattle finished on grass differs from those finished on grain [29]. Hence, to ensure predictive accuracy, the multidimensional fluorescence technique should be used for cattle serum under regulated conditions such as rearing environments and feeding management.
Conclusion
We developed a rapid method to directly measure serum retinol concentrations in cattle using multidimensional fluorescence without the need for sample preparation. This provides a practical and highly accurate way to monitor vitamin A intake in fattening Japanese Black cattle to ensure high-quality meat. As previously reported, the EEMs of food and biological samples have been used to measure various compounds such as amino acids, pigments, and vitamins. Therefore, we are confident that the fluorescence method can be used in livestock health checks to measure multiple metabolites in serum.
Acknowlegements
We thank Mr. Yoichi Kimijima (JA Higashinihon Kumiai Shiryo Co., Japan) for assistance with collecting cattle serum samples and Dr. Yonathan Asikin (University of the Ryukyus) for helpful comments on the manuscript.
Data Availability
No applicable.
References
Gorocica-Buenfil MA, Fluharty FL, Reynolds CK, Loerch SC (2007) Effect of dietary vitamin a concentration and roasted soybean inclusion on marbling, adipose cellularity, and fatty acid composition of beef. J Anim Sci 85:2230–2242. https://doi.org/10.2527/jas.2006-780
Oka A, Dohgo T, Juen M, Saito T (1998) Effects of vitamin a on beef quality, weight gain, and serum concentrations of thyroid hormones, insulin-like growth factor-I, and insulin in Japanese Black steers. Anim Sci Technol 69:90–99. https://doi.org/10.2508/chikusan.69.90
Oka A, Maruo Y, Miki T, Yamasaki T, Saito T (1998) Influence of vitamin A on the quality of beef from the Tajima strain of Japanese Black cattle. Meat Sci 48:159–167. https://doi.org/10.1016/s0309-1740(97)00086-7
Gallina AM, Helmboldt H, Frier HI, Nielsen SW, Eaton HD (1970) Bone growth in the hypovitaminotic A calf. J Nutr 100:129–141. https://doi.org/10.1093/jn/100.1.129
He X, Li Y, Li M, Jia G, Dong H, Zhang Y, He C, Wang C, Deng L, Yang Y (2012) Hypovitaminosis A coupled to secondary bacterial infection in beef cattle. BMC Vet Res 8:222. https://doi.org/10.1186/1746-6148-8-222
Collins CA, Chow CK (1984) Determination of vitamin A and vitamin A acetate by high-performance liquid chromatography with fluorescence detection. J Chromatogr 317:349–354. https://doi.org/10.1016/s0021-9673(01)91674-7
Driskell WJ, Hewett JS, Bashor MM (1986) Evaluation of a direct fluorometric method for determination of serum retinol. Clin Chem 32:867–869. https://doi.org/10.1093/clinchem/32.5.867
Futterman S, Swanson S, Kalina RE (1975) A new, rapid fluorometric determination of retinol in serum. Investig Ophthalmol 14:125–130
Chiba A, Kokawa M, Tsuta M, Todoriki S (2019) Predicting sensory evaluation indices of Cheddar cheese texture by fluorescence fingerprint measurement coupled with an optical fibre. Int Dairy J 91:129–136. https://doi.org/10.1016/j.idairyj.2018.10.001
Lawaetz AJ, Bro R, Kamstrup-Nielsen M, Christensen IJ, Jørgensen LN, Nielsen HJ (2012) Fluorescence spectroscopy as a potential metabonomic tool for early detection of colorectal cancer. Metabolomics 8:111–121. https://doi.org/10.1007/s11306-011-0321-4
Møller JK, Parolari G, Gabba L, Christensen J, Skibsted LH (2003) Monitoring chemical changes of dry-cured Parma ham during processing by surface autofluorescence spectroscopy. J Agric Food Chem 26:1224–1230. https://doi.org/10.1021/jf025662h
Shirshin E, Cherkasova O, Tikhonova T, Berlovskaya E, Priezzhev A, Fadeev V (2015) Native fluorescence spectroscopy of blood plasma of rats with experimental diabetes: identifying fingerprints of glucose-related metabolic pathways. J Biomed Opt 20:051033. https://doi.org/10.1117/1.JBO.20.5.051033
Christensen J, Povlsen VT, Sørensen J (2003) Application of fluorescence spectroscopy and chemometrics in the evaluation of processed cheese during storage. J Dairy Sci 86:1101–1107. https://doi.org/10.3168/jds.S0022-0302(03)73692-3
Christensen J, Miquel-Becker E, Frederiksen CS (2005) Fluorescence spectroscopy and PARAFAC in the analysis of yogurt. Chemom Intell Lab Syst 75:201–208. https://doi.org/10.1016/j.chemolab.2004.07.007
Hougaard AB, Anders JL, Richard HI (2013) Front face fluorescence spectroscopy and multi-way data analysis for characterization of milk pasteurized using instant infusion. LWT 53:331–337. https://doi.org/10.1016/j.lwt.2013.01.010
Ullah R, Khan S, Ali H, Bilal M, Saleem M (2017) Identification of cow and buffalo milk based on Beta carotene and vitamin-A concentration using fluorescence spectroscopy. PLoS One 12:e0178055. https://doi.org/10.1371/journal.pone.0178055
Kang C, Wu HL, Xiang SX, Xie LX, Liu YJ, Yu YJ, Sun JJ, Yu RQ (2014) Simultaneous determination of aromatic amino acids in different systems using three-way calibration based on the PARAFAC-ALS algorithm coupled with EEM fluorescence: exploration of second-order advantages. Anal Methods 6:6358–6368. https://doi.org/10.1039/C4AY00943F
Kang C, Wu HL, Zhou C, Xiang SX, Zhang XH, Yu YJ, Yu RQ (2016) Quantitative fluorescence kinetic analysis of NADH and FAD in human plasma using three- and four-way calibration methods capable of providing the second-order advantage. Anal Chim Acta 910:36–44. https://doi.org/10.1016/j.aca.2015.12.047
Niazi A, Yazdanipour A, Ghasemi J, Abbasi A (2006) Determination of riboflavin in human plasma by excitation-emission matrix fluorescence and multi-way analysis. J Chin Chem Soc 53:503–510. https://doi.org/10.1002/jccs.200600066
Fearn T (2002) Assessing calibrations: SEP, RPD, RER and R2. NIR news 13:12–13. https://doi.org/10.1255/nirn.689
Li X, Sun C, Zhou B, He Y (2015) Determination of hemicellulose, cellulose and lignin in moso bamboo by near infrared spectroscopy. Sci Rep 5:17210. https://doi.org/10.1038/srep17210
Trivittayasil V, Tsuta M, Imamura Y, Sato T, Otagiri Y, Obata A, Otomo H, Kokawa M, Sugiyama J, Fujita K, Yoshimura M (2016) Fluorescence fingerprint as an instrumental assessment of the sensory quality of tomato juices. J Sci Food Agric 96:1167–1174. https://doi.org/10.1002/jsfa.7199
Dufoura E, Frencia JP, Kanea E (2003) Development of a rapid method based on front-face fluorescence spectroscopy for the monitoring of fish freshness. Food Res Int 36:415–423. https://doi.org/10.1016/S0963-9969(02)00174-6
Svensson VT, Andersen CM (2014) EEM fluorescence spectroscopy as a fast method to assess the brine composition of salted herring. LWT 57:775–781. https://doi.org/10.1016/j.lwt.2014.01.030
Ullah R, Khan S, Ali H, Bilal M (2020) Potentiality of using front face fluorescence spectroscopy for quantitative analysis of cow milk adulteration in buffalo milk. Spectrochim Acta A Mol Biomol Spectrosc 225:117518. https://doi.org/10.1016/j.saa.2019.117518
Kamal M, Karoui R (2017) Monitoring of mild heat treatment of camel milk by front-face fluorescence spectroscopy. LWT 79:586–593. https://doi.org/10.1016/j.lwt.2016.11.013
Tian H, Wang W, Zheng N, Cheng J, Li S, Zhang Y, Wang J (2015) Identification of diagnostic biomarkers and metabolic pathway shifts of heat-stressed lactating dairy cows. J Proteome 125:17–28. https://doi.org/10.1016/j.jprot.2015.04.014
Watanabe D, Hase M, Ando T, Ohtsuka H, Miyamoto T, Abe S (2008) Determination of vitamin a in cattle serum using a fluorescence microplate reader (in Japanese with English summary). J Jpn Vet Med Assoc 61:43–47. https://doi.org/10.12935/jvma1951.61.43
Carrillo JA, He Y, Li Y, Liu J, Erdman RA, Sonstegard TS, Songa J (2016) Integrated metabolomic and transcriptome analyses reveal finishing forage affects metabolic pathways related to beef quality and animal welfare. Sci Rep 6:25948. https://doi.org/10.1038/srep25948
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors have no conflict of interests.
Ethical Approval
This study involving animals was in accordance with “standards relating to the care and keeping of industrial animals (Notice of the Prime Minister’s Office No. 22 of 1987)” by the Ministry of the Environment, Government of Japan.
Consent to Participate
No applicable.
Code Availability
No applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tamura, Y., Inoue, H., Takemoto, S. et al. A Rapid Method to Measure Serum Retinol Concentrations in Japanese Black Cattle Using Multidimensional Fluorescence. J Fluoresc 31, 91–96 (2021). https://doi.org/10.1007/s10895-020-02640-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-020-02640-w