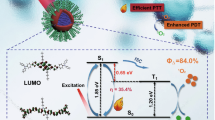
Abstract
New porphyrin analogues have been designed and synthesized using pyrrole, various aldehydes and propionic acid. The formation of desired compounds was analyzed by utilizing the spectral analysis such as IR, NMR and Mass spectroscopy. The studies on absorption and fluorescence emission of synthesized porphyrins were used to evaluate photophysical characteristics such as molar excitation coefficient and Stokes shift. The estimated values of fluorescence lifetime and fluorescence quantum yield of synthesized porphyrins were found to be variable due to the presence of change in the electron donating and withdrawing characters. The efficiency of generation of singlet oxygen by each synthesized porphyrin as photosensitizer was measured in terms of singlet oxygen quantum yield through photooxidation of 9,10-dimethylantharacene. The obtained singlet oxygen quantum yield values were found to be higher in case of porphyrins those have more electron withdrawing characters rather than donating characters as compared to reference 5,10,15,20-tetraphenylporphyrin (H2TPP). The singlet oxygen quantum yield values of synthesized porphyrins varied from 0.52 to 0.66. Pleasingly, some of synthesized porphyrins are found to be photostable and competent to discover as PDT agents as compared to reference H2TPP.





Similar content being viewed by others
References
Fayter D, Corbett M, Heirs M, Fox D, Eastwood A (2010) A systematic review of photodynamic therapy in the treatment of pre-cancerous skin conditions, Barrett’s oesophagus and cancers of the biliary tract, brain, head and neck, lung, oesophagus and skin, health Technol. Assess 14:1–288
Sunar U (2013) Monitoring photodynamic therapy of head and neck malignancies with optical spectroscopies. World J Clin Cases 1:96–105
Liu Y, Wang W, Jianhong Y, Zhou C, Sun J (2013) pH-sensitive polymeric micelles triggered drug release for extracellular and intracellular drug targeting delivery. Asian J Pharmaceu Sci 8:159–167
Shafirstein G, Battoo A, Harris K, Baumann H, Gollnick SO, Lindenmann J, Nwogu CE (2016) Photodynamic therapy of non-small cell lung Cancer. Narrative review and future directions. Ann Am Thorac Soc 13:265–275
Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D (2011) Photodynamic therapy of cancer: an update. CA Cancer J Clin 61:250–281
Brown SB, Brown EA, Walker I (2004) The present and future role of photodynamictherapy in cancer treatment. Lancet Oncol 5:497–508
Jang B, Choi Y (2012) Photosensitizer-conjugated gold Nanorods for enzyme-Activatable fluorescence imaging and photodynamic therapy. Theranostics 2:190–197
Henderson BW, Dougherty TJ (1992) How does photodynamic therapy work? Photochem Photobiol 155:145–157
Josefsen LB, Boyle RW (2012) Unique diagnostic and therapeutic roles of porphyrins and phthalocyanines in photodynamic therapy, imaging and theranostics. Theranostics 2:916–966
Bhaumik J, Gogia G, Kirar S, Vijay L, Thakur NS, Banerjee UC, Laha JK (2016) Bioinspired nanophotosensitizers: synthesis and characterization of porphyrin–noble metal nanoparticle conjugates. New J Chem 40:724–731
Scalise I, Durantini EN (2004) Photodynamic effect of metallo 5- (4-carboxyphenyl)-10,15,20-tris(4-methylphenyl) porphyrins in biomimetic AOT reverse micelles containing urease. J Photochem Photobiol A 162:105–113
Johnson-White B, Zeinali M, Shaffer KM, Patterson CH, Charles PT, Markowitz MA (2007) Detection of organics using porphyrin embedded nanoporous organosilicas. Biosens Bioelectron 22:1154–1162
Jeong EY, Burri A, Lee S, Park SE (2010) Synthesis and catalytic behavior of tetrakis(4-carboxyphenyl) porphyrin-periodic mesoporous organosilica. J Mater Chem 20:10869–10875
Peng Q, Moan J, Ma LW, Nesland JM (1995) Uptake, localization, and photodynamic effect of meso-tetra(hydroxyphenyl)porphine and its corresponding chlorin in normal and tumor tissues of mice bearing mammary carcinoma. Cancer Res 55:2620–2626
Rojkiewicz M, Kus P, Kozub P, Kempa M (2013) The synthesis of new potential photosensitizers [1]. Part 2. Tetrakis-(hydroxyphenyl) porphyrins with long alkyl chain in the molecule. Dyes Pigments 99:627–635
Ormond AB, Freeman HS (2013) Effects of substituents on the photophysical properties of symmetrical porphyrins. Dyes Pigments 96:440–448
Spesia MB, Lazzeri D, Pascual L, Rovera M, Durantini EN (2005) Photoinactivation of Escherichia coli using porphyrin derivatives with different number of cationic charges. FEMS Immunol Med Microbiol 44:289–295
Mahajan PG, Dige NC, Vanjare BD, Phull AR, Kim SJ, Hong SK, Lee KH (2018) Synthesis, photophysical properties and application of new porphyrin derivatives for use in photodynamic therapy and cell imaging. J Fluoresce 28:871–882
Nifiatis F, Athas JC, Gunaratne KDD, Gurung Y, Monette KM, Shivokevich PJ (2011) Substituent effect of porphyrin on singlet oxygen generation quantum yields. The Open Spectroscopy Journal 5:1–12
Mahajan PG, Dige NC, Vanjare BD, Raza H, Mubashir H, Seo SY, Kim CY, Lee KH (2019) Facile synthesis of new quinazolinone benzamides as potent tyrosinase inhibitors: comparative spectroscopic and molecular docking studies. J Mol Struc 1198:126915
Uttamlal M, Holmes-Smith AS (2008) The excitation wavelength dependent fluorescence of porphyrins. Chem Phys Lett 454:223–228
Sun X, Zhang J, He B (2005) The synthesis and photochemical characterization of mesotetra-thienyl porphyrins. J Photochem Photobio A: Chem 172:283–288
Ha JH, Ko S, Lee CH, Lee CY, Kim YR (2001) Effect of core atom modification on photophysical properties and singlet oxygen generation efficiencies: tetraphenylporphyrin analogues core-modified by oxygen and/or sulfur. Chem Phys Lett 349:271–278
Mahajan PG, Dige NC, Desai NK, Patil SR, Kondalkar VV, Hong SK, Lee KH (2018) Selective detection of Co2+ by fluorescent nano probe: Diagostic approach for analysis of environmental samples and biological activities. Spectrochim Acta A: Mol Biomol Spectro 198:136–144
Dige NC, Mahajan PG, Dhokale RK, Chinchkar SM, Patil MV, Pore DM (2019) Novel route for the synthesis of 5-(4-Hydroxy-2- oxo-2H-chromen-3-yl)-1,3-dimethyl-1H-chromeno[2,3-d]pyrimidine-2,4(3H,5H)-diones. Org Prep Proced Int 51:553–565
Serra AC, Pineiro M, Rocha Gonsalves A, Abrantes M, Laranjo M, Santos AC (2008) Halogen atom effect on photophysical and photodynamic characterisics of derivatives of 5,10,15,20-tetrakis(3-hydroxyphenyl)porphyrin. J Photochem Photobio B: Biology 92:59–65
Mahajan PG, Dige NC, Vanjare BD, Eo SH, Seo SY, Kim SJ, Hong SK, Choi CS, Lee KH (2019) A potential mediator for photodynamic therapy based on silver nanoparticles functionalized with porphyrin. J Photochem Photobio A Chemistry 377:26–35
Mahajan PG, Dige NC, Vanjare BD, Phull AR, Kim SJ, Lee KH (2019) Gallotannin mediated silver colloidal nanoparticles as multifunctional nano platform: rapid colorimetric and turn-on fluorescent sensor for Hg2+, catalytic and In vitro anticancer activities. J Lumin 206:624–633
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF- 2019R1I1A3A01059089).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 2965 kb)
Rights and permissions
About this article
Cite this article
Mahajan, P.G., Dige, N.C., Vanjare, B.D. et al. Design and Synthesis of New Porphyrin Analogues as Potent Photosensitizers for Photodynamic Therapy: Spectroscopic Approach. J Fluoresc 30, 397–406 (2020). https://doi.org/10.1007/s10895-020-02513-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-020-02513-2