Abstract
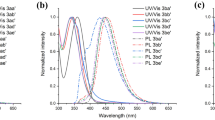
In the present work, novel water-soluble cyclotriphosphazene derivatives (3b and 4b) were synthesized by ‘click’ reactions between cyclotriphosphazene derivative with hydrophilic glycol side groups (2) and Bodipy’s (3a and 4a). All newly synthesized compounds (2, 3b and 4b) were characterized by fourier-transform infrared (FTIR), mass and NMR spectroscopy techniques and elemental analysis (EA). The photophysical properties of Bodipy substituted novel cyclotriphosphazenes (3a and 4a) were examined via UV-Vis absorption and fluorescence emission spectroscopy inside water and many organic solvents such as acetone, tetrahydrofuran, dichloromethane, dimethyl sulfoxide, etc., and the results were compared with the each other.

Graphical Abstract










Similar content being viewed by others
References
Mark JE, Allcock HR, West R (1992) Inorganic polymers. Prentice Hall, Englewood Cliffs
Allcock HR (1972) Phosphorusenitrogen compounds. Academic Press, New York Chapters 6 and 7
Caminade AM, Hameau A, Majoral JP (2016) The specific functionalization of cyclotriphosphazene for the synthesis of smart dendrimers. Dalton Trans 45:1810–1822
Rao MR, Gayatri G, Kumar A, Sastry GN, Ravikanth M (2009) Cyclotriphosphazene ring as a platform for multiporphyrin assemblies. Chem Eur J 15:3488–3496
Yenilmez-Çifçi G, Senkuytu E, Durmus M, Yuksel F, Kılıç A (2013) Fluorenylidene bridged cyclotriphosphazenes: ‘turn-off’ fluorescence probe for Cu2+ and Fe3+ ions. Dalton Trans 42:14916–14926
Coles SJ, Davies DB, Eaton RJ, Hursthouse MB, Kılıç A, Shaw RA, Uslu A (2006) The structural and stereogenic properties of pentaerythritoxy-bridged cyclotriphosphazene derivatives: spiro–spiro, spiro–ansa and ansa–ansa isomers. Dalton Trans 10:1302–1312
Cosut B (2014) Highly efficient energy transfer in BODIPY–pyrene decorated cyclotriphosphazene. Dyes Pigments 100:11–16
Bolink HJ, Santamaria SG, Sudhakar S, Zhen C, Sellinger A (2008) Solution processable phosphorescent dendrimers based on cyclic phosphazenes for use in organic light emitting diodes (OLEDs). Chem Commun 5:618–620
Rao MR, Bolligarla R, Butcher RJ, Ravikanth M (2010) Hexa boron-Dipyrromethene Cyclotriphosphazenes: synthesis, crystal structure, and Photophysical properties. Inorg Chem 49:10606–10616
Uslu A, Kılıç A, Güvenaltın Ş (2010) The investigation of structural and thermosensitive properties of new phosphazene derivative bearing glycol and aminoalcohol. Inorg Chim Acta 363:3721–3726
Uslu A, Kılıç A, Güvenaltın Ş (2010) Structural and thermosensitive properties of novel octopus shape cyclotriphosphazenes. Polyhedron 29:2516–2521
Luten J, van Steenis JH, van Someren R, Kemmink J, Schuurmans-Nieuwenbroek NME, Koning GA, Crommelin DJA, van Nostrum CF, Hennink WE (2003) Water-soluble biodegradable cationic polyphosphazenes for gene delivery. J Control Release 89:483–497
Wilfert S, Iturmendi A, Schoefberger W, Kryeziu K, Heffeter P, Berger W, Brüggemann O, Teasdale I (2014) Water-soluble, biocompatible polyphosphazenes with controllable and pH-promoted degradation behaviour. J Polym Sci A Polym Chem 52:287–294
Christova D, Ivanova SD, Velichkova RS, Tzvetkova P, Mihailova P, Lakov L, Peshev O (2001) New functionalized cyclotriphosphazenes - synthesis and application in the sol-gel process. Des Monomers Polym 4:329–341
Selvaraj II, Chaklanobis S, Chandrasekhar V (1998) New lipophilic cyclo- and poly-phosphazenes containing surfactant substituents. Polym Int 46:111–116
Yenilmez-Çiftçi G, Şenkuytu E, Bulut M, Durmuş M (2015) Novel Coumarin substituted water soluble Cyclophosphazenes as “turn-off” type fluorescence Chemosensors for detection of Fe3+ ions in aqueous media. J Fluoresc 25:1819–1830
Tümay SO, Yıldırım-Sarıkaya S, Yeşilot S (2018) Novel iron(III) selective fluorescent probe based on synergistic effect of pyrene-triazole units on a cyclotriphosphazene scaffold and its utility in real samples. J Lumin 196:126–135
Loudet A, Burgess K (2007) BODIPY dyes and their derivatives: syntheses and spectroscopic properties. Chem Rev 107:4891–4932
Ziessel R, Ulrich G, Harriman A (2007) The chemistry of Bodipy: a new El Dorado for fluorescence tools. New J Chem 31:496–501
Ulrich G, Ziessel R, Harriman A (2008) The chemistry of fluorescent Bodipy dyes: versatility unsurpassed. Angew Chem Int Ed 47:1184–1112
Niu S, Massif C, Ulrich G, Renard PY, Romieu A, Ziessel R (2012) Water-soluble red-emitting Distyryl-Borondipyrromethene (BODIPY) dyes for biolabeling. Chem Eur J 18:7229–7242
Zhu S, Zhang J, Janjanam J, Bi J, Vegesna G, Tiwari A, Luo FT, Wie J, Liu H (2013) Highly water-soluble, near-infrared emissive BODIPY polymeric dye bearing RGD peptide residues for cancer imaging. Anal Chim Acta 758:138–144
Kim J, Kim Y (2014) A water-soluble sulfonate-BODIPY based fluorescent probe for selective detection of HOCl/OCl− in aqueous media. Analyst 139:2986–2989
Chauhan P, Chu K, Yan N, Ding Z (2016) Comparison study of electrochemiluminescence of boron-dipyrromethene (BODIPY) dyes in aprotic and aqueous solutions. J Electroanal Chem 781:181–189
Bura T, Ziessel R (2011) Water-soluble phosphonate-substituted BODIPY derivatives with tunable emission channels. Org Lett 13(12):3072–3075
Xu J, Qian L, Yue Y, Guo Y, Shao S (2014) A water-soluble BODIPY derivative as a highly selective “turn-on” fluorescent sensor for H2O2 sensing in vivo. Biosens Bioelectron 56:58–63
Hooper N, Beeching LJ, Dyke JM, Morris A, Ogden JS, Dias AA, Costa ML, Barros MT, Cabral MH, Moutinho AMC (2002) A study of the thermal decomposition of 2-Azidoethanol and 2-Azidoethyl acetate by ultraviolet photoelectron spectroscopy and matrix isolation infrared spectroscopy. J Phys Chem 106:9968–9975
Liu JY, Yeung HS, Xu W, Li X, Ng DKP (2008) Highly efficient energy transfer in Subphthalocyanine−BODIPY conjugates. Org Lett 10:5421–5424
Çetindere S, Çoşut B, Yeşilot S, Durmuş M, Kılıç A (2014) Synthesis and properties of axially BODIPY conjugated subphthalocyanine dyads. Dyes Pigments 101:234–239
Allcock HR, Bender JD, Marford RV, Berda EB (2003) Synthesis and characterization of novel solid polymer electrolytes based on poly(7-oxanorbornenes) with pendent Oligoethyleneoxy-functionalized Cyclotriphosphazenes. Macromolecules 36:3563–3569
Atilgan S, Ozdemir T, Akkaya EU (2010) Selective Hg(II) sensing with improved stokes shift by coupling the internal charge transfer process to excitation energy transfer. Org Lett 12:4792–4795
Çetindere S, Tümay SO, Kılıç A, Durmuş M, Yeşilot S (2017) Synthesis and physico-chemical properties of cyclotriphosphazene-BODIPY conjugates. Dyes Pigments 139:517–523
Keum D, Kim S, Kim Y (2014) A fluorescence turn-on sensor for the detection of palladium ions that operates through in situ generation of palladium nanoparticles. Chem Commun 50:1268–1270
Kamkaew A, Burgess K (2015) Aza-BODIPY dyes with enhanced hydrophilicity. Chem Commun 51:10664–10667
Erten-Ela S, Yilmaz MD, Icli B, Dede Y, Icli S, Akkaya EU (2008) A panchromatic Boradiazaindacene (BODIPY) sensitizer for dye-sensitized solar cells. Org Lett 10(15):3299–3302
Tümay SO, Yıldırım-Sarıkaya S, Yeşilot S (2018) Novel Iron (III) selective fluorescent probe based on synergistic effect of pyrene-Triazole units on a Cyclotriphosphazene scaffold and its utility in real samples. J Lumin 196:126–135
Uslu A, Tümay SO, Şenocak A, Yuksel F, Özcan E, Yeşilot S (2017) Imidazole/benzimidazole-modified cyclotriphosphazenes as highly selective fluorescent probes for Cu2+: synthesis, configurational isomers, and crystal structures. Dalton Trans 46:9140–9156
Ozay H, Kagit R, Yildirim M, Yesilot S, Ozay O (2014) Novel hexapodal triazole linked to a cyclophosphazene core rhodamine-based chemosensor for selective determination of Hg2+ ions. J Fluoresc 24:1593–1601
Lakowicz JR (2006) Principles of fluorescence spectroscopy, 3rd edn. Springer, Berlin
Qin W, Baruah M, Sliwa M, van der Auweraer M, De Borggraeve WM, Beljonne D, van Averbeke B, Boens N (2008) Ratiometric, fluorescent BODIPY dye with Aza crown ether functionality: synthesis, Solvatochromism, and metal ion complex formation. J Phys Chem A 112:6104–6114
Okutan E, Tümay SO, Yeşilot S (2016) Colorimetric fluorescent sensors for hemoglobin based on BODIPY dyes. J Fluoresc 26:2333–2343
Jacques P, Braun AM (1981) Laser flash photolysis of Phthalocyanines in solution and microemulsion. Helvetica Chimicaacta 64(169):1800–1806
Fery-Forgues S, Lavabre D (1999) Are fluorescence quantum yields so tricky to measure? A demonstration using familiar stationery products. J Chem Educ 76:1260–1264
Acknowledgements
The authors would like to thanks the Gebze Technical University (GTU) for the provided financial support (Grant no: BAP 2017-A105-38).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 2467 kb)
Rights and permissions
About this article
Cite this article
Çetindere, S., Okutan, E., Tümay, S.O. et al. Novel Water-Soluble Cyclotriphosphazene-Bodipy Conjugates: Synthesis, Characterization and Photophysical Properties. J Fluoresc 29, 1143–1152 (2019). https://doi.org/10.1007/s10895-019-02424-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-019-02424-x