Abstract
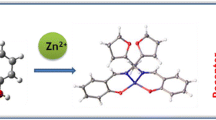
A novel Isophthaloyl-based symmetrical (12E,21E)-N1’,N3’-bis(2-hydroxybenzylidene) isophthalohydrazide, receptor (1) was synthesized and characterized using various spectroscopic technique. The reorganization ability of receptor (1) was evaluated in semi-aqueous medium and shows significant enhancement in fluorescence intensity for Zn (II) ion over various metal ions in CH3CN:H2O (1:1, v/v). The 1:2 binding stoichiometry between receptor (1) and Zn (II) ion was established using Job’s plot and the proposed complex structure was calculated by applying Density Functional Theory (DFT) method. The binding constant (Ka) of receptor (1) with Zn (II) ion was established with the Benesi-Hildebrand plot, Scatchard and Connor’s plot and the values are 1.00 × 104 M−1, 1.05× 104 M−1 and 1.05× 104 M−1 respectively. The limit of detection (LOD) and limit of quantification (LOQ) of receptor (1) and Zn (II) ion was 0.292 μM and 0.974 μM respectively. The binding mode was due to photo-induced electron transfer (PET) and the coordination of Zn (II) ion with C = N hydroxyl group of receptor (1). Electrochemical analysis of metal free receptor (1) and with Zn (II) ion also confirmed the formation of complex.








Similar content being viewed by others
References
Lehn JM (1995) Supramolecular chemistry: concepts and perspectives. Wiley-VCH, Weinheim
Gale PA (2001) Anion receptor chemistry highlights from 1999. Coord Chem Rev 213:79–128. https://doi.org/10.1016/S0010-8545(00)00364-7
Valeur B et al (Eds.) (2001) New trends in fluorescence Spectroscopy_ applications to chemical and life sciences, Part of the Springer Series on Fluorescence book series (SS FLUOR, volume 1)
de Silva AP, Gunaratne HQN, Gunnlaugsson T, Huxley AJM, McCoy CP, Rademacher JT, Rice TE (1997) Signaling Recognition Events with Fluorescent Sensors and Switches. Chem Rev 97:1515–1566. https://doi.org/10.1021/cr960386p
de Silva AP et al (2000) Combining luminescence, coordination and electron transfer for signalling purposes. Coord Chem Rev 205:41–57. https://doi.org/10.1016/S00108545(00)00238-1
Que EL, Domaille DW, Chang CJ (2008) Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem Rev 108(5):1517–1549. https://doi.org/10.1021/cr078203u
Frederickson CJ, Suh SW, Koh J-Y, Cha YK, Thompson RB, LaBuda CJ, Balaji RV, Cuajungco MP (2002) Depletion of intracellular zinc from neurons by use of an extracellular Chelator in vivo and in vitro. J Histochem Cytochem 50(12):1659–1662. https://doi.org/10.1177/002215540205001210
Stewart AJ, Blindauer CA, Berezenko S, Sleep D, Sadler PJ (2003) Interdomain zinc site on human albumin. PNAS 100(7):3701–3706. https://doi.org/10.1073/pnas.0436576100
Perales-Calvo J, Lezamiz A, Garcia-Manyes S (2015) The Mechanochemistry of a Structural Zinc Finger. J Phys Chem Lett 6(17):3335–3340. https://doi.org/10.1021/acs.jpclett.5b01371
Xing G, DeRose VJ (2001) Designing metal–peptide models for protein structure and function. Curr Opin Chem Biol 5:196–200. https://europepmc.org/abstract/med/11282347. Accessed Mar 2018
Lim NC, Freake HC, Bruckner C (2004) Illuminating zinc in biological systems. Chem Eur J 11:38–49. https://www.ncbi.nlm.nih.gov/pubmed/15484196. Accessed Mar 2018
Prasad AS (2008) Zinc in human health: effect of zinc on immune cells. Mol Med 14(5–6):353–357. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2277319/. Accessed Mar 2018
Folin M, Contiero E, Vaselli GM (1994) Zinc content of normal human serum and its correlation with some hematic parameters. Biometals. 7(1):75–79. https://doi.org/10.1007/BF00205198
Shankar AH, Prasad AS (1998) Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr 68(suppl):447S–463S. https://www.ncbi.nlm.nih.gov/pubmed/9701160. Accessed Mar 2018
WHO (2003) Zinc in drinking-water. Background document for preparation of WHO guidelines fordrinking-water quality. World Health Organization (WHO/SDE/WSH/03.04/17), Geneva
U.S. EPA,(May 2009) National Primary Drinking Water Regulations. http://www.epa.gov/safewater/ki.PDF. Accessed Mar 2018
Prasad AS (1998) Zinc and immunity. Mol Cell Biochem 188:63–69. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2277319/. Accessed Mar 2018
Bush AI, Tanzi RE (2002) The galvanization of _-amyloid in Alzheimer’s Disease. PNAS 99(11):7317–7319. https://doi.org/10.1073/pnas.122249699
Larson AA, Kitto KF (1998) J Pharmacol Exp Ther 288(2):759–765. http://jpet.aspetjournals.org/content/288/2/759.short. Accessed Mar 2018
Danscher G, Stoltenberg M (2005) Zinc-specific autometallographic in vivo selenium methods: tracing of zinc-enriched (ZEN) terminals, ZEN pathways, and pools of zinc ions in a multitude of other ZEN cells. J Histochem Cytochem 53(2):141–153. https://doi.org/10.1369/jhc.4R6460.2005
Yörük İ, Deger Y, Mert H, Mert N, Ataseven V (2007) Serum Concentration of Copper, Zinc, Iron, and Cobalt and the Copper/Zinc Ratio in Horses with Equine Herpesvirus-1. Biol Trace Elem Res 118:38–42. https://doi.org/10.1007/s12011-007-0023-y
Bitanihirwe BKY, Cunningham MG (2009) Zinc: The Brain’s Dark Horse bitanihirwe. SYNAPSE 63:1029–1049. https://doi.org/10.1002/syn.20683
Zatta P, Drago D, Bolognin S, Sensi SL (2009) Alzheimer’s disease, metal ions and metal homeostatic therapy. Trends Pharmacol Sci 30(7):346–354. https://doi.org/10.1016/j.tips.2009.05.002
Li W, Wang J, Zhang J, Wang W (2015) Molecular simulations of metal-coupled protein folding. Curr Opin Struct Biol 30:25–31. https://doi.org/10.1016/j.sbi.2014.11.006
Burdette SC, Walkup GK, Spingler B, Tsien RY, Lippard SJ (2001) Fluorescent sensors for Zn2+ based on a fluorescein platform:synthesis, properties and intracellular distribution. J Am Chem Soc 123:7831–7841. https://doi.org/10.1021/ja010059l
Maruyama S, Kikuchi K, Hirano T, Urano Y, Nagano T (2002) A novel, cell-permeable, fluorescent probe for Ratiometric imaging of zinc ion. J Am Chem Soc 124:10650–10651. https://doi.org/10.1021/ja026442n
Callan JF, de Silva AP, Magri DC (2005) Luminescent sensors and switches in the early 21st century. Tetrahedron 61(36):8551–8588. https://doi.org/10.1016/j.tet.2005.05.043
Lee DY, Singh N, Kim MJ, Jang DO (2010) Ratiometric fluorescent determination of Zn(II): a new class of tripodalreceptorusing mixed imine and amide linkages. Tetrahedron 66(2010):7965–7969. https://doi.org/10.1016/j.tet.2010.08.024
Lukowiak B, Vandewalle B, Riachy R, Kerr-Conte J, Gmyr V, Belaich S, Lefebvre J, Pattou F (2001) Identification and purification of functional human beta-cells by a new specific zinc-fluorescent probe. J Histochem Cytochem 49(4):519–528. https://www.ncbi.nlm.nih.gov/pubmed/11259455. Accessed Mar 2018
Nimmanon T, Ziliotto S, Morris S, Flanagana L, Taylor KM (2017) Phosphorylation of zinc channel ZIP7 drives MAPK, PI3K and mTOR growth and proliferation signalling. Metallomics 9:471–481. https://www.ncbi.nlm.nih.gov/pubmed/28205653. Accessed Mar 2018
Aulsebrook ML, Graham B, Grace MR, Tuck KL (2014) The synthesis of luminescent lanthanide-based chemosensorsfor the detection of zinc ions. Tetrahedron 70(29):1–6. https://doi.org/10.1016/j.tet.2014.04.078
Song EJ, Park GJ, Lee JJ, Lee S, Noh I, Kim Y, Kim S-J, Kim C, Harrisond RG (2015) A fluorescence sensor for Zn2+ that also acts as a visible sensor for Co2+ and Cu2+. Sensors Actuators B Chem 213(5):268–275. https://doi.org/10.1016/j.snb.2015.02.094
Stuart Tobias R (1958) The determination of stability constants of complex inorganic species in aqueous solutions. J Chem Educ 35(12):592–599. https://doi.org/10.1021/ed035p592
Benesi HA, Hildebrand JH (1949) A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J Am Chem Soc 71(8):2703–2707. https://doi.org/10.1021/ja01176a030
Scatchard G (1949) The attractions of Protelns for small molecules And ions. Ann N Y Acad Sci 51:660–672. https://doi.org/10.1111/j.1749-6632.1949.tb27297.x
Connors KA (1932) The measurements of molecular complex stability. Wiley, New York, p c1987
MacDougall D, Crummett WB et al (1980) Guidelines for Data Acquisition and Data Quality Evaluation in Environmental Chemistry. Anal Chem 52:2242–2249. https://doi.org/10.1021/ac50064a004
Frisch MJ, Trucks GW, Schlegel HB et al (2004) Gaussian 03, revision C.02. Gaussian Inc, Wallingford CT
Santos-Figueroa LE, Moragues ME, Manuela M, Raposo M et al (2012) Synthesis and evaluation of thiosemicarbazones functionalized with furylmoieties as new chemosensors for anion recognition org. Biomol Chem 10:7418–7428. https://doi.org/10.1039/c2ob26200b
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1564 kb)
Rights and permissions
About this article
Cite this article
Khadke, N.B., Patil, A.A., Patil, D.Y. et al. Isophthaloyl-Based Selective Fluorescence Receptor for Zn (II) Ion in Semi-Aqueous Medium. J Fluoresc 29, 837–843 (2019). https://doi.org/10.1007/s10895-019-02385-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-019-02385-1