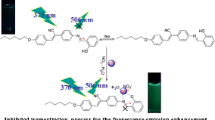
Abstract
The recognition ability of N-Furfurylsalicylaldimine (HL) toward various cations (Pb2+, Hg2+, Ba2+, Cd2+, Ag+, Zn2+, Cu2+, Ni2+, Co2+, K+, Sr2+, and Na+) has been studied by UV–Vis and fluorescence spectroscopy. The compound showed highly selective fluorescence signaling behaviour for Zn2+ ions in methanol-water medium based on CHEF process and is capable of distinguishing Zn2+ from Cd2+ ion. From single crystal X-ray analysis it is revealed that a Zn2+ ion binds two ligand molecules through imine nitrogen and phenolate oxygen atom.

N-Furfurylsalicylaldimine as a selective sensing of Zn2+ ion through CHEF process. The x-ray structure of the receptor-Zn(II) complex shows 2:1 stochiometry









Similar content being viewed by others
References
ThomasIII SW, Joly GD, Swager TM (2007) Chemical sensors based on amplifying fluorescent conjugated polymers. Chem Rev 107:1339–1386
Szacilowski K, Macyk W, Matuszek AD, Brindell M, Stochel G (2005) Bioinorganic photochemistry: frontiers and mechanisms. Chem Rev 105:2647–2694
Vallee BL, Falchuk KH (1993) The biochemical basis of zinc physiology. Physiol Rev 73:79–118
Frederickson CJ, Koh J-H, Bush AI (2005) The neurobiology of zinc in health and disease. Nat Neurosci 6:449–462
Berg JM, Shi YG (1996) The galvanization of biology: a growing appreciation for the roles of zinc. Science 271:1081–1085
Assaf SY, Chung SH (1984) Release of endogenous Zn2+ from brain tissue during activity. Nature 308:734–736
Outten CE, O’Halloran TV (2001) Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 292:2488–2492
Spichiger-Keller US (1998) Chemical sensors and biosensors for medical and biological applications. Wiley-VCH, Weinheim
Nolan EM, Lippard SJ (2008) Tools and tactics for the optical detection of mercuric ion. Chem Rev 108:3443–3480
Xu Z, Xiao Y, Qian X, Cui J, Cui D (2005) Ratiometric and selective fluorescent sensor for CuII based on internal charge transfer (ICT). Org Lett 7:889–892
Gunnlaugsson T, Davis AP, O’Brien JE, Glynn (2002) M fluorescent sensing of pyrophosphate and Bis-carboxylates with charge neutral PET chemosensors. Org Lett 4:2449–2452
Lim NC, Schuster JV, Porto MC, Tanudra MA, Yao L, Freake HC, Bruckner C (2005) Coumarin-based chemosensors for Zinc(II): toward the determination of the design algorithm for CHEF-type and ratiometric probes. Inorg Chem 44:2018–2030
Beer PD (1998) Transition-metal receptor systems for the selective recognition and sensing of anionic guest species. Acc Chem Res 31:71–80
Nishizawa S, Kato Y, Teramae N (1999) Fluorescence sensing of anions via intramolecular excimer formation in a pyrophosphate-induced self-assembly of a pyrene-functionalized guanidinium receptor. J Am Chem Soc 121:9463–9464
Wu J-S, Liu W-M, Zhuang X-Q, Wang F, Wang P-F, Tao S-L, Zhang X-H, Wu S-K, Lee S-T (2007) Fluorescence turn on of coumarinderivatives by metal cations: a new signaling mechanism based on C=N isomerization. Org Lett 9:33–36
Sahana A, Banerjee A, Guha S, Lohar S, Chattopadhyay A, Mukhopadhyay SK, Das D (2012) Highly selective organic fluorescent probe for azide ion: formation of a “molecular ring”. Analyst.137:1544-1546.
Peng X, Wu Y, Fan J, Tian M, Han KJ (2005) Colorimetric and ratiometric fluorescence sensing of fluoride: tuning selectivity in proton transfer. J Org Chem 70:10524–10531
Das S, Guha S, Banerjee A, Lohar S, Sahana A, Das D (2011) 2-(2-Pyridyl) benzimidazole based Co(II) complex as an efficientfluorescent probe for trace level determination of aspartic and glutamic acid in aqueous solution: A displacement approach. Org Biomol Chem 9:7097–7104
Serin JM, Brousmiche DW, Frechet JMJ (2002) A fret-based ultraviolet to near-infrared frequency converter. J Am Chem Soc 124:11848–11849
Majzoub AE, Cadiou C, Olivier ID, Tinant B, Chuburu F (2011) Cyclam-methylbenzimidazole: a Selective OFF-ON Fluorescent Sensor for Zinc. Inorg Chem 50: 4029-4038 and references there in
Song EJ, You JKGR, Park GJ, Kim YKS-J, Kim C, Harrison RG (2013) A single molecule that acts as a fluorescence sensor for zinc and cadmium and a colorimetric sensor for cobalt. Dalton Trans 42:15514–15520
Ganguly A, Paul BK, Ghosh S, Kar S, Guchhait N (2013) Selective fluorescence sensing of Cu(II) and Zn(II) using a new Schiff base-derived model compound: naked eye detection and spectral deciphering of the mechanism of sensory action. Analyst 138:6532–6541
Xiao F, Shen J, Qu J, Jing S, Zhu D-R (2013) A fluorescent sensor with mixed N/O/Se donor atoms for probing Zn(II) ion. Inorg Chem Commun 35:69–71
Hsieh WH, Wanb C-F, Liao D-J, Wua A-T (2012) A turn-on Schiff base fluorescence sensor for zinc ion. Tetrahedron Lett 53:5848–5851
Li L, Dang Y-Q, Li H-W, Wang B, Wu Y (2010) Fluorescent chemosensor based on Schiff base for selective detection of zinc(II) in aqueous solution. Tetrahedron Lett 51:618–621
Kim KB, Kim H, Song EJ, Kim S, Noh I, Kim CA (2013) cap-type Schiff base acting as a fluorescence sensor for zinc(II) and a colorimetric sensor for iron(II), copper(II), and zinc(II) in aqueous media. Dalton Trans 42:16569–16577
Bhagwat UA, Mukhedkar VA, Mukhedkar AJ (1980) Study of ligand isomeric complexes of N-Furfurylsalicylaldimine. J Chem Soc Dalton Trans:2319
Hanaoka K, Kikuchi K, Kojima H, Urano Y, Nagano T (2004) Development of a Zinc ion-selective luminescent lanthanidechemosensor for biological applications. J Am Chem Soc 126:12470–12471
Baek NY, Heo CH, Lim CS, Masanta G, Cho BR, Kim HM (2012) A highly sensitive two-photon fluorescent probe for mitochondrial zinc ions in living tissue. Chem Commun 48:4546–4548
(a) Kubo Y, Kato M, Y Misawa, Tokita S (2004) A fluorescence-active 1,3-bis(isothiouronium)-derived naphthalene exhibiting versatile binding modes toward oxo anions in aqueous MeCN solution: new methodology for sensing oxoanions. Tetrahedron Lett 45:3769-3772. (b) Yang MH, Thirupathi P, Lee KH (2011) Selective and Sensitive Ratiometric Detection of Hg(II) Ions Using a Simple Amino Acid Based Sensor. Org Lett 13:5028-5031
Morris JV, Mahaney MA, Huber JR (1976) Fluorescence quantum yield determinations. 9, l0-diphenylanthracene as a reference standard in different solvents. J Phys Chem 80(9):969–974
Aligent (ed) (2010) CrysAlis PRO. Agilent Technologies Ltd, Yarnton
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) OLEX2: a complete structure solution, refinement and analysis program. J Appl Crystallogr 42:339–341
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr A64:112–122
Acknowledgments
This project was financially supported by CSIR, New Delhi, India [No. 01(2536)/11/EMR-II]. S.K.J. is thankful to UGC for providing research fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Supplementary data
CCDC 945929 contains the supplementary crystallographic data for 1. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336 033; or e-mail: deposit@ccdc.cam.ac.uk. (DOCX 535 kb)
Rights and permissions
About this article
Cite this article
Jana, S.K., Bera, M., Puschmann, H. et al. Sensing of Zn2+ion by N-Furfurylsalicylaldimine Based on CHEF Process†. J Fluoresc 24, 1245–1251 (2014). https://doi.org/10.1007/s10895-014-1407-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-014-1407-y