Abstract
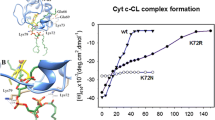
Specific interactions between a mitochondrial hemoprotein cytochrome c (cyt c) and cardiolipin, a lipid component of mitochondrial membrane, are crucial to electron shuttling and apoptotic activities of this protein. In the present study the Förster resonance energy transfer (FRET) between anthrylvinyl-labeled phosphatidylcholine as a donor and heme moiety of cyt c as an acceptor was employed to give a quantitative characterization of the protein binding to the model membranes from the mixtures of phosphatidylcholine (PC) with phosphatidylglycerol (PG), phosphatidylserine (PS) or cardiolipin (CL) in different molar ratios. The multiple arrays of the FRET data were globally analyzed in terms of the model of energy transfer in two-dimensional systems combined with the scaled particle adsorption model. The arguments in favor of the specificity of cyt c interactions with CL were obtained, including the higher adsorption potential and the deeper protein insertion in the lipid bilayer.




Similar content being viewed by others
References
Selvin PR (2000) The renaissance of fluorescence resonance energy transfer. Nature Struct Biol 7:730–734
Sahoo H (2011) Förster resonance energy transfer—a spectroscopic nanoruler: principle and applications. J Photochem Photobiol C 12:20–30
Ma L, Yang F, Zheng J (2014) Application of fluorescence resonance energy transfer in protein studies. J Mol Struct 1077:87–100
Rowland CE, Delehanty JB, Dwyer CL, Medintz IL (2017) Growing applications for bioassembled Förster resonance energy transfer cascades. Mater Today 20:131–141
Loura L, Prieto M (2011) FRET in membrane biophysics: an overview. Frontiers Physiol 92:1–11
Corbalan-Garcia S, Sanchez-Carrillo S, Garcia-Garcia J, Gomez-Fernandez JC (2003) Characterization of the membrane binding mode of the C2 domain of PKCε. BioChemistry 42:11661–11668
Nazarov PV, Koehorst RB, Vos WL, Apanasovich VV, Hemminga MA (2006) FRET study of membrane proteins: simulation-based fitting for analysis of membrane protein embedment and association. Biophys J 91:454–466
Silvius JR, Nabi IR (2006) Fluorescence-quenching and resonance energy transfer studies of lipid microdomains in model and biological membranes. Mol Membr Biol 23:5–16
Calleja V, Ameer-Beg SM, Vojnovic B, Woscholski R, Downward J, Larijani B (2003) Monitoring conformational changes of proteins in cells by fluorescence lifetime imaging microscopy. Biochem J 372:33–40
Chigaev A, Buranda T, Dwyer DC, Prossnitz ER, Sklar LA (2003) FRET detection of cellular α4-integrin conformational activation. Biophys J 85:3951–3962
Yano Y, Takemoto T, Kobayashi S, Yasui H, Sakurai H, Ohashi W, Niwa M, Futaki S, Sugiura Y, Matsuzaki K (2002) Topological stability and self-association of a completely hydrophobic model transmembrane helix in lipid bilayers. BioChemistry 41:3073–3080
You M, Li E, Wimley WC, Hristova K (2005) Förster resonance energy transfer in liposomes: measurements of transmembrane helix dimerization in the native bilayer environment. Anal Biochem 340:154–164
Domanov Y, Gorbenko G, Molotkovsky J (2004) Global analysis of steady-state energy transfer measurements in membranes: resolution of structural and binding parameters. J Fluoresc 14:49–55
Cusanovich MA, Hazzard JT, Meyer TE, Tollin G (1989) Electron transfer mechanisms in heme proteins. J Macromol Sci Chem A26:433–443
Diaz-Moreno I, Garcia-Heredia JM, Diaz-Quitana A, De la Rosa MA (2011) Cytochrome c signalosome in mitochondria. Eur Biophys J 40:1301–1315
Goodsell DS (2004) The molecular perspective: cytochrome c and apoptosis. The Oncologist 9:226–227
Lewis RNA, McElhaney RN (2009) The physicochemical properties of cardiolipin bilayers and cardiolipin-containing lipid membranes. Biochim Biophys Acta 1788:2069–2079
Pinheiro TJT (1994) The interaction of horse heart cytochrome c with phospholipid bilayers. Structural dynamic effects Biochimie 76:489–500
Nantes IL, Zucchi MR, Nascimento OR, Faljoni-Alario A (2001) Effect of heme iron valence state on the conformation of cytochrome c and its association with membrane interfaces. J Biol Chem 276:153–158
Sinibaldi F, Milazzo L, Howes BD, Piro MC, Fiorucci L, Polticelli F, Ascenzi P, Coletta M, Smulevich G, Santucci R (2017) The key role played by charge in the interaction of cytochrome c with cardiolipin. J Biol Inorg Chem 22:19–29
Sinibaldi F, Howes BD, Droghetti E, Polticelli F, Piro MC, Di Pierro D, Fiorucci L, Coletta M, Smulevich G, Santucci R (2013) Role of lysines in cytochrome c–cardiolipin interaction. BioChemistry 52:4578–4588
Kalanhni E, Wallace CJA (2007) Cytochrome c impaled: investigation of the extended lipid anchorage of a soluble protein to mitochondrial membrane models. Biochem J 407:179–187
Tuominen EKJ, Wallace CJA, Kinnunen PKJ (2002) Phospholipid-cytochrome c interaction. Evidence for the extended lipid anchorage. J Biol Chem 277:8822–8826
Hanske J, Toffey JR, Morenz AM, Bonilla AJ, Schiavoni KH, Pletneva EV (2012) Conformational properties of cardiolipin-bound cytochrome c. Proc Natl Acad Sci USA 109:125–130
Muenzner J, Pletneva E (2014) Structural transformations of cytochrome c upon interaction with cardiolipin. Chem Phys Lipids 179:57–63
Pandiscia LA, Schweitzer-Stenner R (2015) Coexistence of native-like and non-native partially unfolded ferricytochrome c on the surface of cardiolipin-containing liposomes. J Phys Chem B 119:1334–1349
Brown L, Wuthrich K (1977) NMR and ESR studies of the interactions of cytochrome c with mixed cardiolipin-phosphatidylcholine vesicles. Biochim Biophys Acta 468:389–410
De Kruijff B, Cullis PR (1980) Cytochrome c specifically induces non-bilayer structures in cardiolipin-containing model membranes. Biochim Biophys Acta 602:477–490
Bergstrom CL, Beales PA, Lv Y, Vanderlick TK, Groves JT (2013) Cytochrome c causes pore formation in cardiolipin-containing membranes. Proc Natl Acad Sci USA 110:6269–6274
Bergelson L, Molotkovsky J, Manevich Y (1985) Lipid-specific probes in studies of biological membranes. Chem Phys Lipids 37:165–195
Molotkovsky J, Smirnova M, Karyukhina M, Bergelson L (1989) Synthesis of anthrylvinyl phospholipids probes. Bioorg Khim 15:377–380
Bulychev AA, Verchoturov VN, Gulaev BA (1988) Current methods of biophysical studies. Vyschaya Shkola, Moscow
Malyshka D, Pandiscia LA, Schwweitzer-Stenner R (2014) Cardiolipin containing liposomes are fully ionized at physiological pH. An FT-IR study of phosphate group ionization. Vib Spectrosc 75:86–92
Chatelier R, Minton AP (1996) Adsorption of globular proteins on locally planar surfaces: models for the effect of excluded surface area and aggregation of adsorbed protein on adsorption equilibria. Biophys J 71:2367–2374
Minton AP (1999) Adsorption of globular proteins on locally planar surfaces. II. Models for the effect of multiple adsorbate conformations on adsorption equilibria and kinetics. Biophys J 76:176–187
Fung BK, Stryer L (1978) Surface density determination in membranes by fluorescence energy transfer. Biochemistry 17:5241–5248
Gorbenko GP, Ioffe VM, Molotkovsky JG, Kinnunen PKJ (2008) Resonance energy transfer study of lysozyme-lipid interactions. Biochim Biophys Acta 1778:1213–1221
Lakowicz JR (2006) Principles of fluorescent spectroscopy. Springer, New York
Dale R, Eisinger J, Blumberg W (1979) The orientational freedom of molecular probes. The orientation factor in intramolecular energy transfer. Biophys J 26:161–194
Eaton WA, Hochstrasser RM (1967) Electronic spectrum of single crystals of ferricytochrome c. J Chem Phys 46:2533–2538
Pinheiro TJT, Watts A (1994) Lipid specificity in the interaction of cytochrome c with anionic phospholipid bilayers revealed by solid-state 31P NMR. Biochemistry 33:2451–2458
Spooner PJR, Watts A (1991) Reversible unfolding of cytochrome c upon interaction with cardiolipin bilayers. 2. Evidence from phosphorus-31 NMR measurements. Biochemistry 30:3880–3885
Stepanov G, Gnedenko O, Mol’nar A, Ivanov A, Vladimirov Y, Osipov A (2009) Evaluation of cytochrome c affinity to anionic phospholipids by means of surface plasmon resonance. FEBS Lett 583:97–100
Hildebrandt P, Heimburg T, Marsh D (1990) Quantitative conformational analysis of cytochrome c bound to phospholipid vesicles studied by resonance Raman spectroscopy. Eur Biophys J 18:193–201
Heimburg T, Hildebrandt P, Marsh D (1991) Cytochrome c-lipid interactions studied by resonance Raman and 31P NMR spectroscopy. Correlation between the conformational changes of the protein and lipid bilayer. BioChemistry 30:9084–9089
Vladimirov YA, Nol’ YT, Volkov VV (2011) Protein-lipid nanoparticles that determine whether cells will live or die. Crystallograph Rep 56:553–559
Cullis PR, de Kruijff B (1979) Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim Biophys Acta 559:399–420
Acknowledgements
This work was supported by the grant No 0116U000937 for Young Scientists from the Ministry of Education Science and of Ukraine.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gorbenko, G.P., Trusova, V. & Molotkovsky, J.G. Förster Resonance Energy Transfer Study of Cytochrome c—Lipid Interactions. J Fluoresc 28, 79–88 (2018). https://doi.org/10.1007/s10895-017-2176-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-017-2176-1