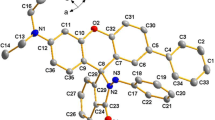
Abstract
A new “turn-on” fluorescent chemosensor based on dansyl derivative was prepared for Cu2+ ion sensing. Hydroxyl, imine and azomethine groups in Schiff base derived compound 1 were deliberately introduced for facilitating the binding of Cu2+ ion. Of screen metal ions, compound 1 showed a high degree of selectivity toward Cu2+ ion. Other interfering metal ions did not affect the fluorescence intensity of compound 1, except Hg2+ and Fe3+ ions exhibited a significant degree of fluorescence quenching. Upon binding of Cu2+ ion, compound 1 displayed a chelation enhanced fluorescence (CHEF) resulting in increasing of the fluorescence intensity. The molecular optimized geometry indicated the binding ratio between compound 1 and Cu2+ ion at 1:1 with the binding constant of 1.68 × 10− 7 M− 1. The optimized condition for sensing ability of compound 1 with a detection limit of 5 × 10− 7 M was found at the physiological pH 7.2 with the excitation wavelength of 366 nm. Due to no cytotoxicity and good photophysical properties, compound 1 was extended its application for the detection of Cu2+ ion in Vero cells. Compound 1 could be potentially used as an intracellular fluorescent chemosensor for tracking Cu2+ ion.

Graphical Abstract













Similar content being viewed by others
References
Hesse L, Beher D, Masters CL, Multhaup G (1994) The βA4 amyloid precursor protein binding to copper. FEBS Lett 349:109–116
Multhaup G, Schlicksupp A, Hess L, Beher D, Ruppert T, Masters CL, Beyreuther K (1996) The amyloid precursor protein of Alzheimer’s disease in the reduction of copper (II) to copper (I). Science 271:1406–1409
Maynard CJ, Bush AI, Masters CL, Cappai R, Li Q-X (2005) Metals and amyloid-β in Alzheimer’s disease. Int J Exp Path 86:147–159
Breslow E (1964) Comparison of cupric ion-binding sites in myoglobin derivatives and serum albumin. J Biol Chem 239:3252–3259
Zgirski A, Frieden E (1990) Binding of Cu(II) to non-prosthetic sites in ceruloplasmin and bovine serum albumin. J Inorg Biochem 39:137–148
Luk CK (1971) Study of the nature of the metal-binding sites and estimate of the distance between the metal-binding sites in transferrin using trivalent lanthanide ions as fluorescent probe. BioChemistry 10:2838–2843
McClure DS (1952) Spin-orbit interaction in aromatic molecules. J Chem Phys 20:682–686
Rurack K, Resch-Genger U, Rettig W (1998) Global analysis of time-resolved emission – a powerful tool for the analytical discrimination of chemically similar ZnII and CdII complexes. J Photochem Photobiol A Chem 118:143–149
Martinez R, Zapata F, Caballero A, Espinosa A, Tarraga A, Molina P (2006) 2-Aza-1,3-butadiene derivatives featuring an anthracene or pyrene unit: highly selective colorimetric and fluorescent signaling of Cu2+ cation. Org Lett 8:3235–3238
Li G-K, Xu Z-X, Chen C-F, Huang Z-T (2008) A highly efficient and selective turn-on fluorescent sensor for Cu2+ ion based on calix[4]arene bearing four iminoquinoline subunits on the upper rim. Chem Commun 1774–1776
Abalos T, Jimenez D, Martines-Manez R, Ros-Lis JV, Royo S, Sancenon F, Soto J, Costero A, Gil M, Parra S (2009) Hg2+ and Cu2+ selective detection using a dual channel receptor based on thiopyrylium scaffoldings. Tetrahedron Lett 50:3885–3888
Ueno A, Minato S, Suzuki I, Fukushima M, Ohkubo M, Osa T, Hamada F, Murai K (1990) Host–guest sensory system of dansyl-modifled β-cyclodextrin for detecting steroidal compounds by dansyl fluorescence. Chem Lett 19:605–608
Wang Y, Ikeda T, Ueno A, Toda F (1992) Syntheses and molecular recognition abilities of 6-O-, 2-O-, and 3-O-dansyl-γ-cyclodextrins. Chem Lett 5:863–866
Hamada F, Kondo Y, Ito R, Suzuki I, Osa T, Ueno A (1993) Dansyl-modified 7-cyclodextrin as a fluorescent sensor for molecular recognition. J Incl Phenom 15:273–279
Wang Y, Ikeda T, Ueno A, Toda F (1994) Dansyl-β-cyclodextrins as fluorescent sensors responsive to organic compounds. Bull Chem Soc Jpn 67:1598–1607
Nakamura M, Ikeda T, Nakamura A, Ikeda H, Ueno A, Toda F (1995) Remarkable molecular recognition of dansyl-modified cyclodextrin dimer. Chem Lett 24:343–344
Nakamura M, Ikeda A, Ise N, Ikeda T, Ikeda H, Toda F, Ueno A (1995) Dansyl-modified β-cyclodextrin with a monensin residue as a hydrophobic, metal responsive cap. J Chem Soc Chem Commun 721–722
Aksuner N, Henden E, Yilmaz I, Cukurovali A (2009) A highly sensitive and selective fluorescent sensor for the determination of copper(II) based on a schiff base. Dyes Pigm 83:211–217
Chen H, Wu Y, Cheng Y, Yang H, Li F, Yang P (2007) A ratiometric fluorescent sensor for zinc(II) with high selectivity. Inorg Chem Commun 10:1413–1415
Sousa C, Gameiro P, Freire C, Castro B (2004) Nickel (II) and copper (II) Schiff base complexes bearing benzo-15-crown-5 functionalities as probes for spectroscopic recognition of lanthanide ions. Polyhedron 23:1401–1408
Bhatt KD, Gupte HS, Makwana BA (2012) Calix receptor edifice; scrupulous turn off fluorescent sensor. J Fluoresc 22:1493–1500
Bhatt KD, Makwana BA, Vyas DJ (2014) Selective recognition by novel calix system: ICT based chemosensor for metal ions. J Lumin 146:450–457
Liu L, Wang A, Wang G, Li J, Zhou Y (2015) A naphthopyran-rhodamine based fluorescent and colorimetric chemosensor for recognition of common trivalent metal ions and Cu2+ ions. Sens Actuators B 215:388–395
Yu MM, Li ZX, Wei LH, Wei DH, Tang MS (2008) A 1,8-naphthyridine-based fluorescent chemodosimeter for the rapid detection of Zn2+ and Cu2+. Org Lett 10:5115–5118
Martinez R, Espinosa A, Tarraga A, Molina P (2010) A new bis(pyrenyl)azadiene-based probe for the colorimetric and fluorescent sensing of Cu(II) and Hg(II). Tetrahedron 66:3662–3667
Franzen S, Ni W, Wang B (2003) Study of the mechanism of electron-transfer quenching by boron-nitrogen adducts in fluorescent sensors. J Phys Chem B 107:12942–12948
Föll RE, Kramer HEA (1990) Role of charge transfer and spin-orbit coupling in fluorescence quenching. a case study with oxonine and substituted benzenes. J Phys Chem 94:2476–2487
Uyanik I, Oguz M, Bhatti AA, Uyanik A, Yilmaz M (2017) A new piperidine derivatized-Schiff base based “turn-on” Cu2+ chemo-sensor. J Fluoresc 27:791–797
Liu Z-C, Yang Z-Y, Li T-R, Wang B-D, Li Y, Qin D-D, Wang M-F, Yan M-H (2011) An effective Cu (II) quenching fluorescence sensor in aqueous solution and 1D chain coordination polymerframework. Dalton Trans 40:9370–9373
García-Beltrán O, Cassels BK, Pérez C, Mena N, Núñez MT, Martínez NP, Pavez P, Aliaga ME (2014) Coumarin-based fluorescent probes for dual recognition of copper (II) and iron (III) ions and their application in bio-Imaging. Sensors 14:1358–1371
Ghiggino KP, Lee AG, Meech SR, O’Connor DV, Phillips D (1981) Time-resolved emission spectroscopy of the dansyl fluorescence probe. BioChemistry 20:5381–5389
Ghisaidoobe ABT, Chung SJ (2014) Intrinsic tryptophan fluorescence in the detection and analysis of proteins: a focus on Förster resonance energy transfer techniques. Int J Mol Sci 15:22518–22538
Chen QY, Chen CF (2005) A new Hg2+ selective fluorescent sensor based on a dansyl amide-armed calix[4]-aza-crown. Tetrahedron Lett 46:165–168
Schonefeld K, Ludwig R, Feller KH (2006) Fluorescence studies of host-guest interaction of a dansyl amide labelled Calix[6]arene. J Fluoresc 16:449–454
Beyeh NK, Aumanen J, Ahman A, Luostarinen M, Mansikkamaki H, Nissinen M, Tommola JK, Rissanen K (2007) Dansylated resorcinarenes. New J Chem 31:370–376
Talanova GG, Talanov VS (2010) Dansyl-containing fluorogenic calixarenes as optical chemosensors of hazardous metal ions: a mini-review. Supramol Chem 22:838–852
Yan J, Fan L, Qin J, Li C, Yang Z (2016) A novel chromone Schiff-base fluorescent chemosensor for Cd (II) based on C = N isomerization. J Fluoresc 26:1059–1065
Vardhan H, Mehta A, Nath I, Verpoort F (2015) Dynamic imine chemistry in metal–organic polyhedra. RSC Adv 5:67011–67030
Ozkan G, Kose M, Zengin H, McKee V, Kurtoglu M (2015) A new Salen-type azo-azomethine ligand and its Ni(II), Cu(II) and Zn(II) complexes: synthesis, spectral characterization, crystal structure and photoluminescence studies. Spectrochim Acta A Mol Biomol Spectrosc 150:966–973
Armbruster DA, Tillman MD, Hubbs LM (1994) Limit of detection (LOD)/limit of quantitation (LOQ): comparison of the empirical and the statistical methods exemplified with GC-MS assays of abused drugs. Clin Chem 40:1233–1238
Williams ATR, Winfield SA, Miller JN (1983) Relative fluorescence quantum yields using a computer controlled luminescence spectrometer. Analyst 108:1067–1071
Oter O, Ertekin K, Kihncarslan R, Ulusoy M, Cetinkaya B (2007) Photocharacterization of a novel fluorescent Schiff base and investigation of its utility as an optical Fe3+ sensor in PVC matrix. Dyes Pigm 74:730–735
Chen Q, Tang S, Jin X, Zou J, Chen K, Zhang T, Xiao X (2009) Investigation of the genotoxicity of quinocetone, carbadox and olaquindox in vitro using Vero cells. Food Chem Toxicol 47:328–334
Williams ATR, Winfield SA, Miller JN (1983) Relative fluorescence quantum yields using a computer-controlled luminescence spectrometer. Analyst 108:1067–1071
Pandey S, Rattan A, Singh M (2011) Evaluation the intermediate results of the QuantiFERON-TB gold in-tube test. Curr Res Tuberc 3:16–19
Chung YM, Raman B, Kim D-S, Ahn KH (2006) Fluorescence modulation in anion sensing by introducing intramolecular H-bonding interactions in host–guest adducts. Chem Commun 186–188
O’Connor NA, Sakata ST, Zhu H, Shea KJ (2006) Chemically modified dansyl probes: a fluorescent diagnostic for ion and proton detection in solution and in polymers. Org Lett 8:1581–1584
Lee MH, Kim HJ, Yoon S, Park N, Kim JS (2008) Metal ion induced FRET OFF – ON in Tren/Dansyl-appended rhodamine. Org Lett 10:213–216
Alam R, Mistri T, Mondal P, Das D, Mandal SK, Khuda-Bukhsh AR, Ali M (2014) A novel copper (II) complex as a nitric oxide turn-on fluorosensor: intracellular applications and DFT calculation. Dalton Trans 43:2566–2576
Zheng Y, Orbulescu J, Ji X, Andreopoulos FM, Pham SM, Leblanc RM (2003) Development of fluorescent film sensors for the detection of divalent copper. J Am Chem Soc 125:2680–2686
Gonzàlez-Jimènez J, Frutos G, Cayre I (1992) Fluorescence quenching of human serum albumin by xanthines. Biochem Pharmacol 44:824–826
Formica M, Fusi V, Giorgi L, Micheloni M (2012) New fluorescent chemosensors for metal ions in solution. Coord Chem Rev 256:170–192
Wu J-S, Liu W-M, Zhuang X-Q, Wang F, Wang P-F, Tao S-L, Zhang X-H, Wu S-K, Lee S-T (2007) Fluorescence turn on of coumarin derivatives by metal cations: a new signaling mechanism based on CN isomerization. Org Lett 9:33–36
Wu J, Liu W, Ge J, Zhang H, Wang P-F (2011) Newsensing mechanisms for design of fluorescent chemosensors emerging in recent years. Chem Soc Rev 40:3483–3495
Karstens T, Kobs K (1980) Rhodamine B and Rhodamine 101 as reference substances for fluorescence quantum yield measurement. J Phys Chem 84:1871–1872
Marsh M, Mcmahon HT (1999) The structural era of endocytosis. Science 9:215–220
Gao H, Yang Z, Zhang S, Cao S, Shen S, Pang Z, Jiang X (2013) Ligand modified nanoparticles increases cell uptake, alters endocytosis and elevates glioma distribution and internalization. Sci Rep 3:1–8
Acknowledgements
Mahidol University grant to P.T. (14/2559) and Development and Promotion of Science and Technology Talents Project’s scholarship (DPST) to W.N. are gratefully acknowledged. We are also grateful to the support of S.S. from National Center for Genetic Engineering and Biotechnology (BIOTEC).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nasomphan, W., Tangboriboonrat, P. & Smanmoo, S. Dansyl Based “Turn-On” Fluorescent Sensor for Cu2+ Ion Detection and the Application to Living Cell Imaging. J Fluoresc 27, 2201–2212 (2017). https://doi.org/10.1007/s10895-017-2161-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-017-2161-8