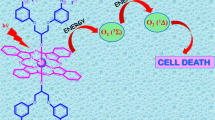
Abstract
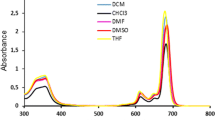
Herein, novel silicon (IV) phthalocyanines peripherally substituted by triethylene glycol groups and bearing axial hydroxyl groups were synthesized and fully characterized by using different analyses techniques. The photophysical and photochemical properties of octa (2a) and tetra (2b) derivatives were investigated in DMF and DMSO. The effect of octa or tetra substitution on fluorescence quantum yield, singlet oxygen generation and photodegradation were examined, and the differences were evaluated regarding their potential efficiency in photodynamic therapy (PDT). Their pH-responses were investigated to determine the influence of protonation of azomethine nitrogen atoms on singlet oxygen generation efficiencies. Dramatic optical changes were observed by protonation of azomethine bridges of 2a and 2b. They exhibited signal decrease from pH 4.0 to 1.0 for 2a (pKa = 2.6) and pH 3.0 to 1.0 for 2b (pKa = 1.8). Besides, the compounds exhibited no aggregation tendency, moderate fluorescence quantum yield, solubility in common organic solvents, high singlet oxygen quantum yield and high photostability in DMF and in DMSO, these favorable properties making them good candidates as photosensitizer for PDT.









Similar content being viewed by others
References
Fan W, Huang P, Chen X (2016) Overcoming the Achilles' heel of photodynamic therapy. Chem Soc Rev 45:6488–6519
Plaetzer K, Krammer B, Berlanda J, Berr F, Kiesslich T (2009) Photophysics and photochemistry of photodynamic therapy: fundamental aspects. Lasers Med Sci 24(2):259–268
Taşkın GC, Durmuş M, Yüksel F, Mantareva V, Kussovski V, Angelov I, Atilla D (2015) Axially paraben substituted silicon(IV) phthalocyanines towards dental pathogen Streptococcus Mutans: synthesis, photophysical, photochemical and in vitro properties. J Photochem Photobiol A Chem 306:31–40
Zhao Z, Chan P-S, Li H, Wong K-L, Wong RNS, Mak N-K, Zhang J, Tam H-L, Wong W-Y, Kwong DW (2011) Highly selective mitochondria-targeting amphiphilic silicon (IV) phthalocyanines with axially ligated rhodamine B for photodynamic therapy. Inorg Chem 51(2):812–821
Taratula O, Schumann C, Naleway MA, Pang AJ, Chon KJ, Taratula O (2013) A multifunctional theranostic platform based on phthalocyanine-loaded dendrimer for image-guided drug delivery and photodynamic therapy. Mol Pharm 10(10):3946–3958
Allison RR, Downie GH, Cuenca R, Hu X-H, Childs CJ, Sibata CH (2004) Photosensitizers in clinical PDT. Photodiagn Photodyn Ther 1(1):27–42
Voon SH, Kiew LV, Lee HB, Lim SH, Noordin MI, Kamkaew A, Burgess K, Chung LY (2014) In vivo studies of nanostructure-based photosensitizers for photodynamic cancer therapy. Small 10(24):4993–5013
He H, Lo P-C, Yeung S-L, Fong W-P, Ng DK (2011a) Synthesis and in vitro photodynamic activities of pegylated distyryl boron dipyrromethene derivatives. J Med Chem 54(8):3097–3102
Gerweck LE (1998) Tumor pH: implications for treatment and novel drug design. Paper presented at the Semin Radiat Oncol
Wang A, Chen X, Zhang L, Zhang G, Zhou L, Lu S, Zhou J, Wei S (2014) Effects of pH on aggregation and photodynamic activities of cationic zinc phthalocyanines substituted with amides. J Photochem Photobiol A Chem 288:1–12
Koo H, Lee H, Lee S, Min KH, Kim MS, Lee DS, Choi Y, Kwon IC, Kim K, Jeong SY (2010) In vivo tumor diagnosis and photodynamic therapy via tumoral pH-responsive polymeric micelles. Chem Commun 46(31):5668–5670
Jiang X-J, Lo P-C, Yeung S-L, Fong W-P, Ng DK (2010a) A pH-responsive fluorescence probe and photosensitiser based on a tetraamino silicon (IV) phthalocyanine. Chem Commun 46(18):3188–3190
Topal SZ, Yuksel F, Gürek AG, Ertekin K, Yenigül B, Ahsen V (2009) Spectroscopic probing of acid–base properties and photocharacterization of phthalocyanines in organic solvents and polymer matrices. J Photochem Photobiol A Chem 202(2):205–213
Jiang XJ, Lo PC, Tsang YM, Yeung SL, Fong WP, Ng DK (2010b) Phthalocyanine–polyamine conjugates as pH-controlled photosensitizers for photodynamic therapy. Chem Eur J 16(16):4777–4783
Ogunsipe A, Nyokong T (2004) Effects of substituents and solvents on the photochemical properties of zinc phthalocyanine complexes and their protonated derivatives. J Mol Struct 689(1):89–97
Topal SZ, Önal E, Gürek AG, Hirel C (2013) pH-induced “off–on–off” type molecular switch behaviors of zinc and free tetraimidazophthalocyanines. Dalton Trans 42(32):11528–11536
Ogunsipe AO, Idowu MA, Ogunbayo TB, Akinbulu IA (2012) Protonation of some non-transition metal phthalocyanines—spectral and photophysicochemical consequences. J Porphyrins Phthalocyanines 16(07n08):885–894
Lau JT, Lo P-C, Jiang X-J, Wang Q, Ng DK (2014) A dual activatable photosensitizer toward targeted photodynamic therapy. J Med Chem 57(10):4088–4097
Perrin D, Armarego W, Perrin D (1980) Purification of laboratory chemicals, 2nd edn. Pergamon Press, New York
Young JG, Onyebuagu W (1990) Synthesis and characterization of di-disubstituted phthalocyanines. J Org Chem 55(7):2155–2159
van Nostrum CF, Picken SJ, Schouten A-J, Nolte RJ (1995) Synthesis and supramolecular chemistry of novel liquid crystalline crown ether-substituted phthalocyanines: toward molecular wires and molecular ionoelectronics. J Am Chem Soc 117(40):9957–9965
Cabezón B, Quesada E, Esperanza S, Torres T (2000) Synthesis of crowned triazolephthalocyanines. Eur J Org Chem 2000(15):2767–2775
Foley S, Jones G, Liuzzi R, McGarvey DJ, Perry MH, Truscott TG (1997) The synthesis and photophysical properties of polyether substituted phthalocyanines of potential use in photodynamic therapy. J Chem Soc Perkin Trans 2(9):1725–1730
Yvon HJ (2012) A guide to recording fluorescence quantum yields. HORIBA, Jobin Yvon Ltd., Stanmore
Williams ATR, Winfield SA, Miller JN (1983) Relative fluorescence quantum yields using a computer-controlled luminescence spectrometer. Analyst 108(1290):1067–1071
Topal SZ, Ertekin K, Gürek AG, Yenigul B, Ahsen V (2011) Tuning pH sensitivities of zinc phthalocyanines in ionic liquid modified matrices. Sensors Actuators B Chem 156(1):236–244
Jacques P, Braun AM (1981) Laser flash photolysis of phthalocyanines in solution and microemulsion. Helv Chim Acta 64(6):1800–1806
Ogunsipe A, Maree D, Nyokong T (2003) Solvent effects on the photochemical and fluorescence properties of zinc phthalocyanine derivatives. J Mol Struct 650(1):131–140
Ogunsipe A, Nyokong T (2005) Photophysical and photochemical studies of sulphonated non-transition metal phthalocyanines in aqueous and non-aqueous media. J Photochem Photobiol A Chem 173(2):211–220
Kuznetsova N, Gretsova N, Kalmykova E, Makarova E, Dashkevich S, Negrimovskii V, Kaliya O, Luk'yanets E (2000) Relationship between the photochemical properties and structure of pophyrins and related compounds. Russ J Gen Chem 70(1):133–140
Seotsanyana-Mokhosi I, Kuznetsova N, Nyokong T (2001) Photochemical studies of tetra-2, 3-pyridinoporphyrazines. J Photochem Photobiol A Chem 140(3):215–222
Jiang X-J, Huang J-D, Zhu Y-J, Tang F-X, Ng DK, Sun J-C (2006) Preparation and in vitro photodynamic activities of novel axially substituted silicon (IV) phthalocyanines and their bovine serum albumin conjugates. Bioorg Med Chem Lett 16(9):2450–2453
Hofman J-W, van Zeeland F, Turker S, Talsma H, Lambrechts SAG, Sakharov DV, Hennink WE, van Nostrum CF (2007) Peripheral and axial substitution of phthalocyanines with solketal groups: synthesis and in vitro evaluation for photodynamic therapy. J Med Chem 50(7):1485–1494
Barker CA, Findlay KS, Bettington S, Batsanov AS, Perepichka IF, Bryce MR, Beeby A (2006) Synthesis of new axially-disubstituted silicon-phthalocyanine derivatives: optical and structural characterisation. Tetrahedron 62(40):9433–9439
Chan CM, Lo P-C, Yeung S-L, Ng DK, Fong W-P (2010) Photodynamic activity of a glucoconjugated silicon (IV) phthalocyanine on human colon adenocarcinoma. Cancer Biol Ther 10(2):126–134
Farren C, Fitz Gerald S, Bryce MR, Beeby A, Batsanov AS (2002) Synthesis, structure and optical characterisation of silicon phthalocyanine bis-esters. J Chem Soc, Perkin Trans 2(1):59–66
Idowu M, Nyokong T (2009) Photophysicochemical and fluorescence quenching studies of tetra-and octa-carboxy substituted silicon and germanium phthalocyanines. J Photochem Photobiol A Chem 204(1):63–68
Maree MD, Kuznetsova N, Nyokong T (2001) Silicon octaphenoxyphthalocyanines: photostability and singlet oxygen quantum yields. J Photochem Photobiol A Chem 140(2):117–125
Nemykin VN, Lukyanets EA (2010) Synthesis of substituted phthalocyanines. ARKIVOC 1:136–208
Ranta J, Viljanen R, Lemmetyinen H, Efimov A (2009) A novel, one-pot method for the synthesis of dimeric and monomeric phthalocyanine silicon complexes from free-base phthalocyanines. Dyes Pigments 83(3):317–323
Büyükekşi SI, Durmuş M, Atilla D (2016) Photophysical and photochemical properties of novel peripherally triethyleneoxysulfonyl substituted monomeric and Si-Si bonded dimeric silicon phthalocyanines. J Porphyrins Phthalocyanines 20(12):1426–1437
Masilela N, Idowu M, Nyokong T (2009) Photophysical, photochemical and electrochemical properties of water soluble silicon, titanium and zinc phthalocyanines. J Photochem Photobiol A Chem 201(2):91–97
Topal SZ, Ün ŞŞ, Bretonnière Y, Kostakoğlu ST (2017) New designed naphthalimide-phthalocyanine pentads: synthesis, photophysical and photochemical properties in DMSO and room temperature ionic liquids. J Photochem Photobiol A Chem 332:562–570
Ghani F, Kristen J, Riegler H (2012) Solubility properties of unsubstituted metal phthalocyanines in different types of solvents. J Chem Eng Data 57(2):439–449
Ogunsipe A, Chen J-Y, Nyokong T (2004) Photophysical and photochemical studies of zinc (II) phthalocyanine derivatives—effects of substituents and solvents. New J Chem 28(7):822–827
İşci Ü, Beyreis M, Tortik N, Topal SZ, Glueck M, Ahsen V, Dumoulin F, Kiesslich T, Plaetzer K (2016) Methylsulfonyl Zn phthalocyanine: a polyvalent and powerful hydrophobic photosensitizer with a wide spectrum of photodynamic applications. Photodiagn Photodyn Ther 13:40–47
Zorlu Y, Dumoulin F, Durmuş M, Ahsen V (2010) Comparative studies of photophysical and photochemical properties of solketal substituted platinum (II) and zinc (II) phthalocyanine sets. Tetrahedron 66(17):3248–3258
Şahin B, Topal SZ, Atilla D (2017) Synthesis, Photophysical and photochemical properties of a set of silicon phthalocyanine bearing antiinflamatuar group. J Fluoresc 27:407–416
Carloni P, Damiani E, Greci L, Stipa P, Tanfani F, Tartaglini E, Wozniak M (1993) On the use of 1, 3-diphenylisobenzofuran (DPBF). Reactions with Carbon and Oxygen Centered Radicals in Model and Natural Systems Res Chem Intermed 19(5):395–405
Acknowledgements
The Scientific Research Council of Gebze Technical University is gratefully acknowledged for the funding through the project (GYTE 2012-A-11).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(DOCX 1.15 mb)
Rights and permissions
About this article
Cite this article
Büyükekşi, S.I., Topal, S.Z. & Atilla, D. Novel Silicon Phthalocyanines Bearing Triethylene Glycol Groups: Photophysical and Photochemical Properties as well as pH-Induced Spectral Behaviour. J Fluoresc 27, 1257–1266 (2017). https://doi.org/10.1007/s10895-017-2057-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-017-2057-7