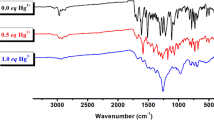
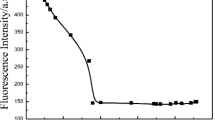
Abstract
Two rhodamine derivatives, N-mono-maleic acid amide-N′-rhodamine B hydrazide (MRBH) and N-mono-succinic acid amide-N′-rhodamine 6G hydrazide (SR6GH), were synthesized by amidation with maleic anhydride (MAH), succinic anhydride (SAH) and rhodamine B hydrazide, rhodamine 6G hydrazide, which were identified by FTIR, 1H NMR and elemental analysis. Two water-soluble fluorescent materials (PVA-MRBH and PVA-SR6GH) were prepared via esterification reaction with N-mono-maleic acyl chloride amide-N′-rhodamine B hydrazide (MRBHCl) or N-mono-maleic acyl chloride amide-N′-rhodamine 6G hydrazide (SR6GHCl) and poly(vinyl alcohol) (PVA) in DMSO solution. The sensing behaviors of PVA-MRBH and PVA-SR6GH were explored by recording the fluorescence spectra in completely aqueous solution. Upon the addition of Cu2+ and Fe3+ ions to the aqueous solution of PVA-MRBH, visual color change from rose pink to amaranth and orange for Cu2+ and Fe3+ ions, respectively, and fluorescence quenching were observed. Titration of Cu2+, Fe3+, Cr3+ or Hg2+ into the aqueous solution of PVA-SR6GH, although they induced fluorescence enhancement, only Fe3+ made the color changing from colorless to yellow. Moreover, other metal ions did not induce obvious changes to color and the fluorescence spectra.









Similar content being viewed by others
References
Li LQ, Meng LP (2014) Novel rhodamine derivate as high selective detection lead sensor. Spectrochim Acta A Mol Biomol Spectrosc 58:772–775
Liu WY, Li HY, Lv HS, Zhao BX, Miao JY (2012) A rhodamine chromene-based turn-on fluorescence probe for selectively imaging Cu2+ in living cell. Spectrochim Acta A Mol Biomol Spectrosc 95:658–663
Sun CD, Chen JM, Ma H, Liu Y, Zhang JH, Liu QJ (2011) A new Cu2+-induced color reaction of a rhodamine derivative N-(3-carboxy)acryloyl rhodamine B hydrazide. Sci China Chem 54(7):1101–1108
Kumari N, Dey N, Bhattacharya S (2014) Remarkable role of positional isomers in the design of sensors for the ratiometric detection of copper and mercury ions in water. RSC Adv 9:4230–4238
Karthigeyan DMD, Kundu TK, Govindaraju T (2013) FRET-based rational strategy for ratiometric detection of Cu2+ and live cell imaging. Sensors Actuator B Chem 176:831–837
Fang XX, Zhang SF, Zhao GY, Zhang WW, Xu JW, Ren AM, Wu CQ, Yang W (2014) The solvent-dependent binding modes of a rhodamine-azacrown based fluorescent probe for Al3+ and Fe3+. Dyes Pigments 101:58–66
Chereddy NR, Suman K, Korrapati PS, Thennarasu S, Mandal AB (2012) Design and synthesis of rhodamine based chemosensors for the detection of Fe3+ ions. Dyes Pigments 95:606–613
Chai MM, Zhang D, Wang M, Hong HJ, Ye Y, Zhao YF (2012) Four rhodamine B-based fluorescent chemosensor for Fe3+ in aqueous solution. Sensors Actuators B Chem 174:231–236
Wang BY, Guan XL, Hu YL, Su ZX (2008) Synthesis and photophysical behavior of a water-soluble fluorescein-bearing polymer for Fe3+ ion sensing. J Polym Res 15:427–433
Ju HY, Lee MH, Kim J, Kim JS, Kim J (2011) Rhodamine-based chemosensing monolayers on glass as a facile fluorescent “turn-on” sensing film for selective detection of Pb2+. Talanta 83:1359–1363
Wan XX, Liu HY, Yao S, Liu TQ, Yao YW (2014) A Stimuli-responsive nanogel-based sensitive and selective fluorescent sensor for Cr3+ with thermo-induced tunable detection sensitivity. Macromol Rapid Commun 35:323–329
Kim HN, Lee MH, Kim HJ (2008) A new trend in rhodamine-based chemosensors: application of spirolactam ring-opening to sensing ions. Chem Soc Rev 37:1465–1472
Bejia M, Afonso CAM, Martinho JMG (2009) Synthesis and applications of rhodamine derivatives as fluorescent probes. Chem Soc Rev 8:2410–2433
Chen X, Wang X, Wang S, Shi W, Wang K, Ma H (2008) A highly selective and sensitive fluorescence probe for the hypochlorite. Chem Eur J 14:4719–4724
Boyarskiy VP, Belov VN, Medda R, Hell SW (2008) Photostable, amino reactive and water-soluble fluorescent labels based on sulfonated rhodamine with a rigidized xanthene fragment. Chem Eur J 14:1784–1792
Dujols V, Ford F, Czarnik AW (1997) A long-wavelength fluorescent chemodosimeter selective for Cu (II) ion in water. J Am Chem Soc 119:7386–7387
Chen X, Pradhan T, Wang F, Kim JS, Yoon J (2012) Fluorescent chemosensors based on spiroring-opening of xanthenes and related derivatives. Chem Rev 112:1910–1956
Hamid EK, Pedro E, Saturnino I, Félix CG, Felipe S, Fouad BB, José MG (2013) Chromogenic and fluorogenic detection of cations in aqueous media by means of an acrylic polymer chemosensor with pendant rhodamine-based dyes. Dyes Pigments 96:414–423
Liu YH, Meng LZ, Lu XJ, Zhang LF, He YB (2008) Thermo and pH sensitive fluorescent polymer sensor for metal cations in aqueous solution. Polym Adv Technol 19:137–143
Wanichacheva N, Praikaew P, Suwanich T, Sukrat K (2014) “Naked-eye” colorimetric and “turn-on” fluorometric chemosensors for reversible Hg(2+) detection. Spectrochim Acta A Mol Biomol Spectrosc 118:908–914
Liu T, Liu SY (2011) Responsive polymers-based dual fluorescent chemosensors for Zn2+ ions and temperatures working in purely aqueous media. Anal Chem 8:32775–32785
Niamsa N, Kaewtong C, Srinonmuang W, Wanno B, Pulpokab B, Tuntulani T (2013) Hybrid organic–inorganic nanomaterial sensors for selective detection of Au3+ using rhodamine-based modified polyacrylic acid (PAA)-coated FeNPs. Polym Chem 4:3039–3046
Lee SH, Parthasarathy A, Schanze KS (2013) A sensitive and selective mercury (II) sensor based on amplified fluorescence quenching in a conjugated polyelectrolyte/spiro-cyclic rhodamine system. Macromol Rapid Commun 34:791–795
Geng TM, Wang Y, Huang RY (2014) Fluorescence sensors for selective detection of Hg2+ ion using a water-soluble poly(vinyl alcohol) bearing rhodamine B moieties. J Fluoresc 24:1207–1213
Geng TM, Wu DY, Huang W, Huang RY, Wu GH (2014) Fluorogenic detection of Hg2+, Cd2+, Fe2+, Pb2+ cations in aqueous media by means of an acrylamide-acrylic acid copolymer chemosensor with pendant rhodamine-based dyes. J Polym Res: 21354–21361
Geng TM, Huang RY, Wu DY (2014) Turn-on fluorogenic and chromogenic detection of Fe3+ and Cr3+ in a completely water medium with polyacrylamide covalently bonding to rhodamine B using diethylenetriamine as a linker. RSC Adv 4(86):46332–46339
Fan JC, Chen J, Yang LM, Lin H, Cao FQ (2009) Preparation of dual-sensitive graft copolymer hydrogel based on N-maleoyl-chitosan and poly(N-isopropylacrylamide) by electron beam radiation. Bull Mater Sci 32:521–526
Yu YQ, Li YS, Liu LX, Zhu CJ, Xu Y (2011) Synthesis and characterization of pH and thermoresponsive Poly(N-isopropylacrylamideco-itaconic acid) hydrogels crosslinked with N-maleyl chitosan. J Polym Res 18:283–291
Yan FY, Cao DL, Wang M, Yang N, Yu QH, Dai LF, Chen L (2012) A new rhodamine-based “Off-On” fluorescent chemosensor for Hg (II) ion and its application in imaging Hg (II) in living cells. J Fluoresc 22:1249–1256
Wang BY, Guan XL, Hu YL, Su ZX (2007) Y Preparation and fluorescent properties of poly(vinyl alcohol) bearing coumarin. Polym Adv Technol 18:529–534
Luo J, Jiang SS, Qin SH, Wu HQ, Wang Y, Jiang JQ, Liu XY (2011) Highly sensitive and selective turn-on fluorescent chemosensor for Hg2+ in pure water based on a rhodamine containing water-soluble copolymer. Sensors Actuators B Chem 160:1191–1197
Yang XF, Guo XQ, Zhao YB (2002) Development of a novel rhodamine-type fluorescent probe to determine peroxynitrite. Talanta 57:883–890
Xiang Y, Tong AJ, Jin PY, Ju Y (2006) New fluorescent rhodamine hydrazone chemosensor for Cu (II) with high selectivity and sensitivity. Org Lett 8:2863–2866
Huang W, Song CX, He C, Lv GJ, Hu XY, Zhu X, Duan CY (2009) Recognition preference of rhodamine-thiospirolactams for mercury (II) in aqueous solution. Inorg Chem 48:5061–5072
Yang YK, Yook KJ, Tae J (2005) A rhodamine-based fluorescent and colorimetric chemodosimeter for the rapid detection of Hg2+ ions in aqueous media. J Am Chem Soc 127:16760–16761
Dong ZP, Yang B, Jin J, Li J, Kang HW, Zhong X, Ri L, Ma JT (2009) Quinoline group modified carbon nanotubes for the detection of zinc ions. Nanoscale Res Lett 4:335–340
Hu QM, Huang GS, Zheng J, Su H, Guo C (2012) Synthesis and rheological properties of hydrophobically modified poly(vinyl alcohol). J Polym Res 19:1–9
Kumar KS, Ramakrishnappa T, Balakrishna RG, Pandurangappa M (2014) A fluorescent chemodosimeter for Hg2+ based on a spirolactam ring-opening strategy and its application towards mercury determination in aqueous and cellular media. J Fluoresc 24:67–74
Kempahanumakkagaari SK, Thippeswamy R, Malingappa P (2014) A new rhodamine B based fluorometric chemodosimeter for Cu2+ ion in aqueous and cellular media. J Lumin 146:11–17
Zhang D, Li M, Wang M, Wang JH, Yang X, Ye Y, Zhao YF (2013) A rhodamine-phosphonate off–on fluorescent sensor for Hg2+ in natural water and its application in live cell imaging. Sensors Actuators B Chem 177:997–1002
Wang M, Yan FY, Zou Y, Chen L, Yang N, Zhou XG (2014) Recognition of Cu2+ and Hg2+ in physiological conditions by a new rhodamine based dual channel fluorescent probe. Sensors Actuators B Chem 192:512–521
Kim KN, Choi MG, Noh JH, Ahn S, Chang SK (2008) Rhodamine B hydrazide revisited: chemodosimetric Hg2+-selective signaling behavior in aqueous environments. Bull Korean Chem Soc 29:571–574
He L, So VLL, Xin JH (2014) A new rhodamine-thiourea/Al3+ complex sensor for the fast visual detection of arginine in aqueous media. Sensors Actuators B Chem 192:496–502
Yuan L, Lin WY, Xie YN, Chen B, Zhu SS (2012) Single fluorescent probe responds to H2O2, NO, and H2O2/NO with three different sets of fluorescence signals. J Am Chem Soc 134:1305–1315
Popescu I, Airinei A, Suflet DM, Popa MI (2011) Maleic acid–2–vinylna phthalene copolymer in aqueous solution: investiga tion of the dissociation and fluorescence quenching. J Polym Res 18:2195–2203
Kaya İ, Kamacı M (2013) Highly selective and stable florescent sensor for Cd(II) based on poly(azomethine-urethane). J Fluoresc 23:115–121
Gao W, Yang YT, Huo FJ, Yin CX, Xu M, Zhang YB, Chao JB, Jin S, Zhang SP (2014) An ICT colorimetric chemosensor and a non-ICT fluorescent chemosensor for the detection copper ion. Sensors Actuators B Chem 193:294–300
Hu ZQ, Du M, Zhang LF, Guo FY, Liu MD, Li M (2014) A novel colorimetric and fluorescent chemosensor for cyanide ion in aqueous media based on a rhodamine derivative in the presence of Fe3+ ion. Sensors Actuators B Chem 193:439–443
Acknowledgments
We acknowledge financial support from the National Natural Science Foundation of China (under Grant No. 21307002, 21407004).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 1322 kb)
Rights and permissions
About this article
Cite this article
Geng, TM., Wang, X., Wang, ZQ. et al. Effects of Single and Double Bonds in Linkers on Colorimetric and Fluorescent Sensing Properties of Polyving Akohol Grafting Rhodamine Hydrazides. J Fluoresc 25, 409–418 (2015). https://doi.org/10.1007/s10895-015-1528-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-015-1528-y