Abstract
Fluorescent dye 2-[(2-Hydroxyethyl)-(1,3-diphenyl-1H-pyrazolo[3,4-b]quinolin-6-ylmethyl)-amino]ethanol (LL1) was examined for its efficiency in the detection of small inorganic cations (lithium, sodium, barium, calcium, magnesium, cadmium, lead and zinc). The dye was synthesized in the laboratory and investigated by means of both, steady-state and time-resolved fluorescence techniques. This compound acts as a fluorescent sensor suitable for detection of small inorganic cations (lithium, sodium, barium, calcium, magnesium, cadmium, lead and zinc) in strongly polar solvent (acetonitrile). An electron transfer from the electro-donative part (receptor) of the molecule to the acceptor part (fluorophore) is thought to be the main mechanism that underlies functionality of the compound as a sensor. This process can be retarded upon complexation of the receptor moiety by inorganic cations. Relatively high sensitivity but poor selectivity of the aminoalcohol thatcontains indicator towards the two-valued cations was observed. However, upon addition of some amounts of water the selectivity of this sensor has been enhanced (especially towards lead cation). The preliminary results in analytical application of the sensor are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Measurements of the cation concentration are of considerable interest and the subject of various investigations conducted by chemists, biologists, clinical biochemists and environmentalists. Some of the cations play a crucial role in biological processes (Na+, K+, Ca2+, Mg2+, Zn2+). In medicine some diagnostics are based on the detection of certain cations and monitoring their concentration in blood and urine. In psychiatry the control of the lithium concentration is important for patients who are under the treatment for manic depression. Some cations are toxic (Pb2+, Cd2+, Hg2+) and early detection of their presence in organism is of uttermost importance.
Among other sensors the fluorescent molecular sensors (known also as indicators) are of considerable interest for the detection of small amounts of cations such as H+, Li+, Na+, K+, Ca2+, Mg2+, Zn2+, Pb2+, Al3+, Cd2+, etc. These are the molecular compounds that fluorescence emission can be strongly affected by the presence of inorganic cations. Their high detection sensitivity permits the investigation of non-fluorescent cations with the concentrations down to 10−6 M [1, 2]. Nevertheless, searches for new fluorescence materials capable of extended sensitivity are still underway.
The principles underlying functionality of fluorescence sensors are presented in several publications including monographs and original papers [3, 4]. Beneath, only the basic information necessary to understand the performance of the sensors is briefly reviewed.
A general principle of operation of many PET fluorescent indicators is based on significant enhancement of fluorescence emission upon addition of cations into fluorescing medium. Molecule-cation complexes of various stoichiometry can be formed in this process. Their high stability in both, the ground and excited states is desired to permit fluorescence emission followed the electronic excitation of the complex (see Scheme 1). To understand fluorescence emission enhancement a detailed mechanism of the process has to be understood. For the purpose of current investigation we recall the mechanism determined for the molecular sensors based on pyrazoloquinoline skeleton. The mechanism underlying functionality of molecular sensors that are constructed on the basis of pyrazoloquinoline skeleton was discussed in the previous papers [5, 6]. It involves an electron transfer process, i.e. after excitation, the pyrazoloquinoline derivative accepts one electron from the lone pair located at the nitrogen atom of the donor receptor moiety, being simultaneously a cation receptor subunit. It was found that previously constructed fluorescent cation indicators are more sensitive to the presence of bivalent cations such as Mg2+, Ca2+, Zn2+ than to monovalent cations such as Li+ and Na+. Additionally, the binding constants estimated from the concentration profiles of the fluorescence changes are related to the charge density of the cations, so that larger was the cation charge density, greater was the complex-formation equilibrium constant.
We chose 1,3-diphenyl-1H-pyrazolo[3,4-b]quinoline as a fluorophore because of its good emissive properties. The fluorescence quantum yields and lifetimes are relatively independent on solvent polarity and reach values up to 88 % and 25 ns, respectively [7]. This fact results in effective photoinduced electron transfer quenching in pyrazoloquinoline compounds with electronically decoupled amino-recognition group and in consequence easily detectable ON-OFF green fluorescence. Another reason for selecting the pyrazoloquinoline is a relatively simple synthesis. 1,3-diphenyl-1H-pyrazolo[3,4-b]quinoline is received in reaction between commercially available substrates. In view of the above, it is not surprising that pyrazoloquinolines are extensively exploited for a long time as a luminescent materials for fabrication of organic light emitting diodes (OLED) [8], as dopants in PVK matrices [9] and polymers [10].
The paper investigates photophysical properties of the chelating sensor 2-[(2-Hydroxyethyl)-(1,3-diphenyl-1H-pyrazolo[3,4-b]quinolin-6-ylmethyl)-amino]ethanol (LL1) and the complexes formed between the sensor and several cations such as Li+, Na+, Ba2+, Ca2+, Mg2+, Cd2+, Pb2+, Zn2+, Ag+ in acetonitrile and acetonitrile-water mixtures as well. To elucidate the potential of LL1 for sensitive and selective detection of cations we perform simple fluorimetric titrations. Additionally, we examine also a possible application of the dye (LL1) as an indicator in fluorimetric determination of Zn and Pb bivalent cations by chloride anion titration in acetonitrile.
Experimental
Synthesis of 2-[(2-Hydroxyethyl)-(1,3-Diphenyl-1H-Pyrazolo[3,4-b]Quinolin-6-Ylmethyl)-Amino]Ethanol (LL1)
The synthesis reaction steps of LL1 are depicted in Scheme 2. 6-methyl-1,3-diphenyl-1H-pyrazolo[3,4-b]quinoline 1 was obtained from the respective pyrazole and aniline precursors [11]. The resulting compound 2 was brominated with NBS (N-bromosuccinimide) in the presence of catalytic amount of AIBN (2,2′-azoisobutyronitrile). The nucleophilic substitution of the bromomethyl group with diethanolamine in acetonitrile gave the compound LL1. A more detailed description of the synthesis of this sensor was given in previous publication [12].
Apparatus and Measuring Procedures
The solvents: dibutyl ether (DBE), butyl chloride (BuCl), tetrahydrofuran (THF), acetone (ACE), acetonitrile (ACN), and dimethylformamide (DMF) were of spectroscopic grade and were used as received (all from Aldrich). All the solvents used in this study did not show any traces of fluorescence. For fluorescence quantum yield and fluorescence lifetime measurements the solutions of the dye were degassed using multiple freeze-pump-thaw cycles. The sample concentration of the dyes (LL1 and fluorophore) for spectroscopic measurements was ca. 4 × 10−5 M (this corresponds to absorbance of ca. 0.2 at the excitation wavelength used for the fluorescence investigations). Lithium, sodium, barium, magnesium, calcium (tetrahydrate) lead (x-hydrate), cadmium, zinc (hexahydrate) perchlorate, and zinc chloride, tetrabutylammonium chloride (TBACl) (Aldrich) were used as received. In an independent measurement it was found that a small addition of deionised water could not influence significantly the fluorescence of the dye alone in acetonitrile solution. The fluorescence titration experiments were not proceeded by the degassing procedure.
The fluorescence measurements of the titration between LL1-Pb2+ complex and tetrabutylammonium chloride (TBACl) were carried out on ultracentrifuged samples. The others fluorimetric titrations were performed in situ.
The absorption spectra were recorded using a Shimadzu UV-2101 PC spectrometer and the emission spectra (with the correction for spectral sensitivity) were measured by means of Hitachi F7000 fluorometer. For time-resolved fluorescence measurements (time-correlated single photon counting technique) a picosecond diode laser (λ = 400 nm, 70 ps pulse duration) (IBH-UK) was used as the excitation source. For steady-state fluorescence measurements a 365 or 380 nm line was used. The fluorescence quantum yield measurements were carried out with quinine sulphate in water (Φ fl = 0.51) [13] as an actinometer.
Joe-Jones Method
The equilibrium constants (K 1) for complexation of the ligand by alkali cations are calculated by fitting Eq. 1 to the relative fluorescence intensities in the presence of different concentration (c M) of the employed alkaline metal perchlorates [14],
where Φ(0), Φ(∞) and Φ(cM) is the fluorescence intensity of the dye alone, with a large concentration of the salt and with the c M concentration of the salt, respectively, c L indicates the analytical concentration of the ligand.
For 2:1 complexation the binding constant K 2 is defined by Eq. 2 [1]:
The Eq. 1 converts into more complex one for 2:1 complexation scheme [3, 4]:
where x denotes the concentration of the complex ML +2 . This quantity can be obtained numerically by solving the third degree polynomial:
with the following parameters:
Job’s Method
Stoichiometry of the fluorescent complexes can be established by measuring the fluorescence of the mixtures of fluorophore (sensor) (L) and metal cations (M) provided that the sum of the concentration of the sensor and the cation is constant, i.e. c L + c M = C. This method is called Job’s method or method of continuous variations [1].
Let us assume the following equilibrium:
We measure the fluorescence of the mixtures at different values of the parameter x, defined as
The position of the maximum of fluorescence on x is related to l/m.
If we assume the 1:1 stoichiometry of a complex the dependence of the integrated fluorescence on x has a following form:
The meaning of the parameters which appeared in Eq. 4a has been explained in the previous section, except of Φ 0(1 − x) defined as the fluorescence intensity measured in the absence of cation at every concentration of the sensor.
The values of the binding constants K 1 and K 2 were obtained from the fluorescence intensities using a nonlinear least-square fitting routine coded in FORTRAN.
Results and Discussion
UV–vis Absorption and Fluorescence Emission Spectroscopy Studies
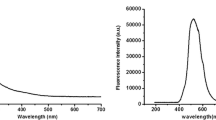
The absorption spectrum of LL1 in acetonitrile consists of two separate bands located between 250 and 325 nm and above 350 nm, respectively (Fig. 1). The first one is a structured band with a maximum at 260 nm (ε = 57400 dm3mol−1cm−1) while the second one is a Gaussian-like band having maximum at 397 nm (molar absorption coefficient ε = 7400 dm3mol−1cm−1). A simple comparison of the absorption spectra of LL1 and the parent molecule 1,3-diphenyl-1H-pyrazolo[3,4-b]quinoline (fluorophore of LL1) reveals a negligible effect of the electron-donating macrocyclic recognition unit on the energetics of the optical transitions within the fluorophore. For instance, the maxima of the absorption and fluorescence bands of LL1 in acetonitrile were found at 397 nm and 481 nm, whilst those of the fluorophore are centred at 395 nm 476 nm (see Fig. 1). It can also be inferred that the absorption spectra of LL1 are rather insensitive to the solvent polarity.
Polarity of the solvents strongly influences the fluorescence lifetimes and quantum yields of LL1, likewise the previously investigated other dyes containing pyrazoloquinoline skeleton [3, 4]. Such dependences of the above mentioned fluorescence quantities on the dielectric permittivity parameter (εs) are tabulated in Table 1.
The reduced quantum yield of LL1 in acetonitrile (Φ fl = 0.04) as compared to the fluorophore part (Φ fl = 0.75 - determined experimentally) and simultaneous decrease of Φ fl and τ fl with increasing solvent polarity suggest the existence of quenching via an electron-transfer process from the electronically decoupled nitrogen atom, located at diethanolamine, to the fluorophore.
The fluorescence decays are monoexponential in nonpolar and medium polar solvents. However, in highly polar solvents the decays are clearly biexponential which may indicate the existence of two different ground-state conformers, related to the endo/exo isomerism [15]. The differences in position and direction of the lone pair at the nitrogen atom and its degree of pyramidalization can be responsible for various electron transfer quenching activities.
A small bathochromic shift of the fluorescence maximum of LL1, observed during the solvatochromic studies, points to a partial charge-transfer character of the transition within the pyrazoloquinoline fragment.
Stability of the Fluorescent Complex (LL1Men+) in Acetonitrile
Because of the large reduction of the fluorescence lifetime and quantum yields reduction, there is an opportunity to apply the molecule LL1 as a PET fluorescent probe in highly polar solvents (dielectric permittivity larger than 7). Acetonitrile was chosen as a solvent for all measurements conducted in this investigation owing to its high dielectric constant, lack of hydrogen bond formation and good solubility of the inorganic salts used in the experiments. Thus, all the results described in this paper are related to the acetonitrile solutions.
Upon addition of cations to the acetonitrile solution of LL1, the absorption spectrum does not change significantly (Fig. 1). The structured band in the UV region (250–325 nm) turns now into a relatively broad band.
However, the addition of cations changes the fluorescence properties considerably (Fig. 2.). The locations of the band maximum in the fluorescence spectra of solutions containing and not containing inorganic cations are only slightly different (shifted bathochromically in the presence of the cations by ca. 10 nm, see inset in Fig. 1) but upon addition of the cations the intensity of the fluorescence rises dramatically (Fig. 2). For example, in the presence of 10 equivalent of Mg2+, the mixture shows an intense green fluorescence and a 10-fold enhancement in the fluorescence intensity at 490 nm.
Similar behaviour has been observed in the experiments when other cations were added. The examples of the titration curves are presented in Fig. 3.
a Dependence of the integrated fluorescence intensity of LL1 as a function of magnesium, barium and silver perchlorate concentration in acetonitrile. The solid line represent the values calculated from Eq. 3 with the association constant K 2 = 7.9 × 108 M−2. b Job’s plot of Ba2+ versus ([LL1] + [Ba2+] =80 μM) at 490 nm emission wavelength and presentation of the fitted function (4a) to experimental data
It can be noticed that the bivalent cations have stronger influence on the fluorescence than the monovalent ones. The values of the binding constants that were calculated from the salt concentration dependencies of the integrated fluorescence intensities, are collected in Table 2. The stoichiometry of the complexes have been established by the analysis of the Job’s plots obtained from the fluorimetric titration of the mixtures of the acetonitrile solutions of the dye (LL1) and that of perchlorates (Fig. 3b). It has been found that in the case of Mg or Zn cation the 2:1 complexes are formed in the ground state. For the other cations the LL1 forms complexes with 1:1 stoichiometry.
One important conclusion emerges from the data collected in Table 2: the compound LL1 in acetonitrile exhibits a great sensitivity but poor selectivity to the presence of bivalent cations such as Mg2+, Zn2+, Cd2+ and Pb2+. Moreover, the equilibrium constants is found in good agreement with charge density of the cation by taking into account the complexes with alkaline and transition metals separately. For practical applications, the detection limit for this sensor evaluated by fluorescence titration was estimated at 4 × 10−7 M, and is 100 times lower than that of the ligand concentration.
Stability of the Fluorescent Complex (LL1Men+) in Acetonitrile-Water Mixtures
The selectivity of the LL1 can be improved by performing fluorimetric measurements in acetonitrile-water mixtures. By adding small amounts of water to the acetonitrile solution of the complex LL1 with magnesium cation can quench fluorescence of the complex almost completely (Fig. 4). The fluorescence of the magnesium complex was immediately quenched in the mixture containing 0.1 molar fraction of water (B), while the complexes with other bivalent cations are almost unaffected and the fluorescence intensity stays the same as in pure acetonitrile (A). However, a systematic increase of water concentration quenches fluorescence of other complexes except of the Pb2+-LL1 complex (C). This is probably the result of different ability of the cations to hydrate.
Hence, a simple addition of water to the mixture of the cations can change the selectivity of the dye making it more prone to form complex only with Pb2+ cation.
Application of LL1 to the Fluorimetric Titration of Cations
The fluorimetric titration of LL1-Pb2+ complex by tetrabutylammonium chloride (TBACl) was performed in acetonitrile. Addition of TBACl to the solution of Pb(ClO4)2 causes a precipitation of PbCl2 with a residue that is hardly soluble in acetonitrile. Further addition of Cl- anions dissolves the precipitate due to formation of the anion complex Pb(Cl4)2- [18]. The titration process can be monitored by fluorescence techniques in the presence of nonfluorescent LL1 dye (Fig. 5). Both PbCl2 and Pb(Cl4)2- cannot form a fluorescent complex with LL1.
Dependence of the integrated fluorescence of the mixture of LL1 (4 × 10−5M) and Pb(ClO4)2 (1 × 10−3M) on the volume of the added TBACl (concentration 2 × 10−2M). Initial volume of the mixture of LL1 and Pb(ClO4)2 is 1 ml. Inset: the first derivative of the titration curve with a minimum (equivalence point) c.a. 0.09 ml of TBACl
As shown in Fig. 5, up to the equivalence point the chloride anions consume mostly the lead cations that are non-bonded to the sensor (being in an excess), thus the fluorescence intensity remains nearly constant. In the vicinity of the equivalence point, the fluorescent complex reacts with the excess of the chloride anions as follows:
and further
Due to the progressive reaction, the concentration of the fluorescent complex LL1-Pb2+ decreases which results in the reduction of fluorescence intensity. Hence, the concentration of the lead cations may be calculated from the equivalence point, that can be determined by analysis of the first derivative of titration curve (Fig. 5 - inset)
Another example of the fluorimetric titration is the reaction of zinc perchlorate (Zn(ClO4)2) with TBACl in acetonitrile. In this system a precipitation of the zinc chloride, that is soluble in acetonitrile, is not observed but the product reacts with an excess of chloride anions to form an anion complex (ZnCl4)2- [19]. Again, this anion does not form a fluorescent complex with the LL1 dye. The fluorimetric titration profile is displayed in Fig. 6.
Dependence of the integrated fluorescence of the mixture of LL1 (4 × 10−5M) and Zn(ClO4)2 (1 × 10−3M) on the volume of the added TBACl (concentration 2 × 10−2M). Initial volume of the mixture of LL1 and Zn(ClO4)2 is 1 ml. Inset: the first derivative of the titration curve with a minimum (equivalence point) c.a. 0.11 ml of TBACl
In this case, the titration curve illustrating the reaction between Zn(LL1)2 2+ and chlorides is more complex. Up to the equivalence point chloride anions reacts only with free zinc cations non-bonded to the LL1 dye (being in excess). Thus the fluorescence intensity remains nearly constant. At the equivalence point it is very likely that the following reaction occurs:
and the fluorescence intensity is reduced up to c.a. 50 % of the initial level. This assumption was proven in the independent titration of LL1 by ZnCl2. It was found out that ZnCl2 can be effectively bound to a chelate (K 1 is equal 2.28 x104 M−1) and likewise in the first equivalence point (Fig. 6), the intensity of the fluorescence (at the high ZnCl2 concentration) is almost twice lower than the maximal intensity of the Zn(LL1)2 2+ complex (both experiments were performed at the same concentration of the dye).
Further addition of chloride anions leads to the formation of an anion complex (ZnCl4)2− and weakly fluorescent LL1 dye. The process has been depicted in scheme 3.
Again, this hypothesis was confirmed by the reaction of ZnCl2 with TBACl in the presence of LL1 dye (Fig. 7)
The present investigation show analytical capabilities of applied fluorescence technique. It can be used for instance for monitoring of a simple exchange reaction where product could be traced by the fluorimetric method. Moreover, the fluorimetric titration experiment shows also that the binding of Zn2+ or Pb2+ to the investigated sensor is reversible in the presence of Cl- anions, which is of particular importance if the sensor will be widely employed in the detection of specific analysis.
Conclusions
The photophysical properties of 2-[(2-Hydroxyethyl)-(1,3-diphenyl-1H-pyrazolo[3,4-b]quinolin-6-ylmethyl)-amino]ethanol (LL1) relevant to that of fluorescent indicator of inorganic cations were investigated. It was found that this dye exhibits a large sensitivity with respect to bivalent cations such as Mg2+, Zn2+, Cd2+ and Pb2+ and its selectivity to detection of bivalent cations can be easily improved by addition of a small amount of water.
The fluorimetric titration (precipitation of hardly soluble lead chloride) using the LL1 dye was performed. The results of the investigation show that this fluorimetric method can be promising for sensitive and selective analysis of cationic species. Its potential importance for application in analytical chemistry and in a range of environmental analyses shall be stressed as well.
References
Valeur B (2002) Molecular fluorescence. Principles and applications. Wiley-VCH, Weinheim, pp 273–348
Prasanna de Silva A, Gunaratne HQN, Gunnlaugsson T, Huxley AJM, McCoy CP, Rademacher JT, Rice TE (1997) Signaling recognition events with fluorescent sensors and switches. Chem Rev 97:1515–1566
Fabbrizzi L, Poggi A (1995) Sensors and switches from supramolecular chemistry. Chem Soc Rev 24:197–202
Bryan AJ, Prasanna de Silva A, de Silva SA, Dayasiri Rupasinghe AD, Samankumara Sandanayake KRA (1989) Photo-induced electron transfer as a general design logic for fluorescent molecular sensors for cations. Biosensors 4:169–179
Mac M, Uchacz T, Wróbel T, Danel A, Kulig E (2010) New fluorescent sensors based on 1H-pyrazolo[3,4-b]quinoline skeleton. J Fluoresc 20:525–532
Mac M, Uchacz T, Danel A, Danel K, Kolek P, Kulig E (2011) New fluorescent sensors based on 1H-pyrazolo[3,4-b]quinoline skeleton. Part II. J Fluoresc 21:375–383
Mac M, Uchacz T, Andrzejak M, Danel A, Szlachcic P (2007) Photophysical properties of some donor- acceptor 1H-pyrazolo[3,4-b]quinolines: Radiative versus non-radiative electron transfer processes. J Photochem Photobiol A 187:78–86
Pan J-H, Chou Y-M, Chiu H-L, Wang B-C (2005) Theoretical investigation of pyrazolo[3,4-b]quinoline derivatives as emitting materials. Tamkang J Sci Eng 8:175–183
He Z, Milburn GHW, Danel A, Puchała A, Tomasik P, Rasała D (1997) Blue electroluminescence of novel pyrazoloquinoline and bispyrazolopyridine derivatives in doped polymer matrices. J Mater Chem 7:2323–2325
Danel A, He Z, Milburn GHW, Tomasik P (1999) Synthesis and characterization of novel photoactive polymer poly(vinyl alcohol)-graft-poly(vinyl naphthalene). J Mater Chem 9:339–342
Tomasik P, Tomasik D, Abramovitch R (1983) Friedlander condensation of 1H-Pyrazolin-5-ones with o-Aminobenzaldehydes. Synthesis of 1H-Pyrazolo[3,4-b]quinolines. J Heter Chem 20:1539–43
Rurack K, Danel A, Rotkiewicz K, Grabka D, Spieles M, Rettig W (2002) 1,3-Diphenyl-1H-pyrazolo[3,4b]quinoline: a versatile fluorophore for the design of brightly emissive molecular sensors. Org Lett 4:4647–4650
Velapoldi RA (1972), Natl. But. Std. 378, Proc. Conf. NBS, Gaithersburg, p. 231
Bourson J, Pouget J, Valeur B (1993) Ion-responsive fluorescent compounds. Effect of cation binding on the photophysical properties of a coumarin linked to monoaza- and diaza-crown ethers. J Phys Chem 97:4552–4557
Buschmann HJ (1987) In: Bernal I (ed) Stereochemical and stereophysical behaviour of macrocycles. Elsevier, Amsterdam, p 103
Barkici H, Koner AL, Nau WM (2005) Binding of inorganic cations by p-sulfonatocalix[4]arene monitored through competitive fluorophore displacement in aqueous solution. Chem Commun 5411–5413
Leray I, Lefevre JP, Delouis JF, Delaire J, Valuer B (2001) Synthesis and photophysical and cation-binding properties of mono- and tetranaphthylcalix[4] arenes as highly sensitive and selective fluorescent sensors for sodium. Chem Eur 7:4590–4598
Hayashha T, Bartsch RA, Kurosawa T, Igawa M (1991) Selective concentration of lead(II) chloride complex with liquid anion-exchange membranes. Anal Chem 63:1023–1027
Mackenzie JD, Murphy WK (1960) Structure of glass-forming halides. II. Liquid zinc chloride. J Phys Chem 33:366–369
Acknowledgments
Drs Andrzej Turek and Marek Tulej are acknowledged for fruitful discussions and help in planning the experiments. This work was partly financed by the basic funding of the University of Agriculture (DS 3133/KCh/2012).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Mac, M., Uchacz, T., Danel, A. et al. Applications of Fluorescent Sensor Based on 1H-pyrazolo[3,4-b]quinoline in Analytical Chemistry. J Fluoresc 23, 1207–1215 (2013). https://doi.org/10.1007/s10895-013-1251-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-013-1251-5