Abstract
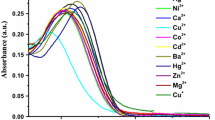
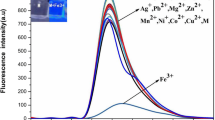
A new fluorescent probe for Mn2+ ion, (6E)-N-((E)-1,2-diphenyl-2-(pyridin-2-ylimino)ethylidene)pyridin-2-amine (L), has been synthesized from benzil and 2-amino pyridine and characterized. In 1:1 (v/v) CH3CN:H2O (pH 4.0, universal buffer) L exhibits fluorescent intensity with emission peak at λmax 360 nm on excitation with photons of 310 nm. Fluorescent intensity of L increases distinguishingly on interaction with Mn2+ ion compared to metal ions—Na+, K+, Ca2+, Mg2+, Ba2+, Fe2+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+, Hg2+, Pb2+ and Ag+ individually or all together. The enhancement in fluorescent intensity is due to snapping of photoinduced electron transfer (PET) prevailed in free L. Fluorescence and UV/visible spectral data analysis shows that binding stoichiometry between Mn2+ and L is 1:1 with log β ≈ 3.0. Both L and its Mn2+ complex were optimised using density functional theory (DFT) and vibrational frequency calculations confirm that both are at local minima on the potential energy surfaces.







Similar content being viewed by others
References
Emsley J (2001) Nature’s building blocks: An a-z guide to the elements. Oxford University Press, Oxford, pp 249–253
Law NA, Caudle MT, Pecoraro VL (1998) Manganese redox enzymes and model systems: properties, structures, and reactivity. Adv Inorg Chem 46:305–440
Dismukes GC, Willigen RTV (2006) Manganese: The oxygen-evolving complex & models. Encyclopedia of Inorganic Chemistry. Princeton University, Princeton
Takeda A (2003) Manganese action in brain function. Brain Res Rev 41:79–87
Bird ED, Anton AH, Bullock B (1984) The effect of manganese inhalation on basal ganglia dopamine concentrations in rhesus monkeys. Neurotoxicology 5:59–66
Gupta SK, Murthy RC, Chandra SV (1980) Neuromelanin in manganese-exposed primates. Toxicol Lett 6:17–20
Furst A (1978) Tumorigenic effect of an organomanganese compound on F344 rats and Swiss albino mice: brief communication. J Natl Cancer Inst 60:1171–1173
Young RJ, Critchley J, Young KK, Freebairn RC, Reynolds AP, Lolin YI (1996) Fatal acute hepatorenal failure following potassium permanganate ingestion. Hum Exp Toxicol 15:259–261
Bouchard MF, Sauve S, Barbeau B, Legrand M, Brodeur M, Bouffard T, Limoges E, Bellinger DC, Mergler D (2010) Intellectual impairment in school-age children. Environ Heal Perspect 119:138–143
Elsner RJF, Spangler JG (2005) Neurotoxicity of inhaled manganese: public health danger in the shower? Med Hypotheses 65:607–616
Cersosimo MG, Koller WC (2006) The diagnosis of manganese-induced parkinsonism. NeuroToxicology 27:340–346
Lu CS, Huang CC, Chu NS, Calne DB (1994) Levodopa failure in chronic manganism. Neurology 44:1600–1602
Lee HN, Xu ZC, Kim SK, Swamy KMK, Kim Y, Kim SJ, Yoon JY (2007) Pyrophosphate -selective fluorescent chemosensor at physiological pH: formation of a unique excimer upon addition of pyrophosphate. J Am Chem Soc 129:3828–3829
Wang JB, Qian XH (2006) Two regioisomeric and exclusively selective Hg(II) sensor molecules composed of a naphthalimide fluorophore and an o phenylenediamine derived triamide receptor. Chem Commun 109–111
Komatsu K, Urano Y, Kojima P, Nagano TJ (2007) Development of an iminocoumarin- based zinc sensor suitable for ratiometric fluorescence imaging of neuronal zinc. J Am Chem Soc 129:13447–13454
Goswami P, Das DK (2012) A new highly sensitive and selective fluorescent cadmium sensor. J Fluoresc 22:391–395
Goswami P, Das DK (2012) N, N, N, N-tetradentate macrocyclic ligand based selective fluorescent sensor for zinc (II). J Fluoresc 22:1081–1085
Zhang J, Campbell RE, Ting AY, Tisen RY (2002) Creating new fluorescent probes for cell biology. Nat Rev Mol Cell Biol 3:906–918
Shuhong X, Wang C, Zhang H, Sun Q, Wang Z, Cui Y (2012) Discriminative detection of bivalent Mn ions by a pH-adjustable recognition method via quantum dot fluorescence sensing. J Mater Chem 22:9216–9221
Gruppi F, Liang J, Bartelle B, Royzen M, Turnbull DH, Canary JW (2012) Supramolecular metal displacement allows on-fluorescence analysis of manganese(II) in living cells. Chem Commun 48:10778–10780
Liang J, Canary JW (2010) Discrimination between hard metals with soft ligand donor atoms: an on fluorescence probe for Manganese (II). Angew Chem Int Ed 49:7710–7713
Haugland RP (2002) Molecular probes: Handbook of fluorescent probes and research products, 9th edn, Molecular Probes
Delly B (1990) An all–electron numerical method for solving the local density functional for polyatomic molecules. J Chem Phys 1990:508–514
Kulatilleke CP, Silva SA, Eliav Y (2006) A coumarin based fluorescent photoinduced electron transfer cation sensor. Polyhedron 25:2593–2596
Goswami P, Baruah S, Das DK (2010) 2,7-Dichlorofluorescein, a fluorescent sensor to detect Cd2+ over Na+, K+, Ca2+, Cu2+, Ni2+ and Zn2+. Indian J Chem 49A:1617–1620
Dutta K, Das DK (2012) 2,7-Diferrocenyl-3,6-diazaocta-2,6-diene: a highly selective dual fluorescent sensor for Zn2+ and Ag+ and electrochemical sensor for Zn2+. Indian J Chem 51A:816–820
Baca SG, Siminov YA, Gerbeleu NV, Gdaniec M, Bourosh PN, Timco GA (2001) Synthesis and X-ray diffraction study of Zn(II) complexes with o-phthalic and aromatic amines. Polyhedron 20:831
Acknowledgment
Department of Science & Technology, New Delhi and University Grants Commission (SAP), New Delhi are thanked for financial help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dutta, K., Deka, R.C. & Das, D.K. A New On-fluorescent Probe for Manganese (II) Ion. J Fluoresc 23, 1173–1178 (2013). https://doi.org/10.1007/s10895-013-1248-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-013-1248-0