Abstract
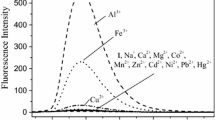
Firstly, a novel pyrazole-pyrazoline fluorescent probe was developed and synthesized. The probe can be used to determine Fe3+ ions in a series of cations in tetrahydrofuran aqueous solution with high selectivity and high sensitivity. After the addition of iron ions, the fluorescence intensity is significantly reduced, Its structure was characterized by 1H NMR, 13C NMR and HR-ESI-MS. UV absorption spectra and Fluorescence spectroscopy were used to study the selective recognition of probe M on metal ions. The probe M can selectivity and sensitivity to distinguish the target ion from other ions through different fluorescence phenomena. In addition, the binding modes of M with Fe3+ were proved to be 1:1 stoichiometry in the complexes by Job’s plot, IR results. The combination of probe M and iron ions is 1:1, and the detection limit is 3.9 × 10−10 M. The binding mode and sensing mechanism of M with Fe3+ was verified by theoretical calculations using Gaussian 09 based on B3LYP/6-31G(d) basis.












Similar content being viewed by others
References
DeSilva AP, Gunaratne HQN, Gunnlaugsson T et al (1997) The stability of cyclodextrin complexes in solution. Chem Rev 97:1514–1566
Aisen P, Wessling-Resnick M, Leibold EA (1999) Iron metabolism Curr Opin. Chem Biol 3:200–206
Touati D (2000) Iron and oxidative stress in bacteria. Arch Biochem Biophys 373:1–6
Bharath S, Hsu M, Kaur D, Rajagopalan S, Andersen JK (2002) Glutathione iron and Parkinsons disease. Biochem Pharmacol 64(5):1037–1048
Honda K, Casadesus G, Petersen RB et al (2004) Oxidative stress and redox-active iron in alzheimers disease. Ann N Y Acad Sci 1012(1):179–182
Narayanaswanmy N, Govindaraju T (2012) Aldazine-based colorimetric sensors for Cu2+ and Fe3+. Chemical 161(1):304–310
Liang ZO, Wang CX, Yang JX, et.al. A highly selective colorimetric chemosensor for detecting the respective amounts of iron(I1) and iron (I1) irons in water. New J Chem 2007;31(6):906–910
Gonzáles APS, Firmino MA, Nomura CS, Rocha FRP, Oliveira PV, Gaubeur I (2009) Peat as a natural solid-phase for copper preconcentration and determination in a multicommuted flow system coupled to flame atomic absorption spectrometry. Anal Chim Acta 636(2):198–194
Mashhadizadeh MH, Pesteh M, Talakesh M, Sheikhshoaie I, Ardakani MM, Karimi MA Solid phase extraction of copper (II) by sorption on octadecyl silica membrane disk modified with a new Schiff base and determination with atomic absorption spectrometry. Spectrochimica Acta Part B Atomic Spectroscopy 2008;63(8):885–8, 888
Liu J, Yu M, Wang XC, Zhang Z (2012) A highly selective colorimetric sensor for Hg2+ based on nitrophenyl-aminothiourea. Spectrochim Acta A 93(10):245–249
Gupta VK, Singh AK, Mergu N (2014) Antipyrine based schiff bases as turn-on fluorescent sensors for Al (III) ion. Electrochim Acta 117(4):405–412
Liu Y, Liang P, Guo L (2005) Nanometer titanium dioxide immobilized on silica gel as sorbent for preconcentration of metal ions prior to their determination by inductively coupled plasma atomic emission spectrometry. Talanta 68(1):25–30
Ferreira SLC, Queiroz AS, Fernandes MS, dos Santos HC (2002) Application of factorial designs and doehlert matrix in optimization of experimental variables associated with the preconcentration and determination of vanadium and copper in seawater by inductively coupled plasma optical emission spectrometry. Spectrochimica Acta Part B Atomic Spectroscopy 57(12):1939–1950
Tesfaldef ZO, Vanstaden JF, Stefan RI (2004) Sequential injecting spectrophotometric determination of irons as Fe(II) in multi-vitamin preparations using 1, 10-phenanthroline as complexing agent. Talanta 64(5):1189–1195
Gomes D, Segundo M, Lima J et al (2005) Spectrophotometric determination of iron and boron in soil extracts using a multi-syringe flow injecting system. Talanta 66(3):703–711
Lunvongsa S, Oshima M, Motomizu S (2006) Determination of total and dissolved amount of iron in water samples using catalytic spectrophotometric flow injecting analysis. Talanta 68(3):969–973
Mohadesi A, Taher MA. Voltammetric determination of cu(II) in naturalwaters and human hair at a meso-2,3-dimercaptosuccinic acid self-assembled gold electrode. Talanta 2007;72(1):95–0, 100
Sahoo SK, Sharma D, Bera RK et al (2012) Iron (III) selective molecular and supramolecular fluorescent probes. Chem Soc Rev 41(21):7195–7227
Levai A, Jeko J (2006) Synthesis of 1-Substituted 5-Aryl-3-styryl-2-pyrazolines and 3-Aryl-5-styryl-2-pyrazolines by the Reaction of Dibenzylideneacetones and E.E-Cinnamylideneacetophenones with Hydrazines. J Heterocyclic Chem 43:1303–1309
Wiley KH (1967) Pyrazles, Pyrazolines, Pyrazilidnes, Indazoles and Condensed rings. The Chemistry of Heterocyclic Compounds 20:180–188
Levai A (2002) Synthesis of 3-Aroyl-4-(3-chromony)-2-pyrazolines. J Heterocyclic Chem 39:1333–1336
Khali HZ, Yanni SA (1981) Synthesis of new Anilido-Pyrazoline and Isoxazoline Derivatines. J Indian Chem Soc 58:168–170
Rawal AA, Thakor VM, Shah NM (1963) Synthesis of some 1, 3,5-Triphenyipyrazolines and 3,5-Diphenyl-cyclohexen-1-ones. J Indian Chem Soc 40(4):323
Dhal PN, Acharya TE, Nayak A (1975) Studies on 1,3-Diaryl Pyrazolines and their derivatives. J Indian Chem Soc 52:1196–1198
Bhatia MS, Sood RK (1978) New heterocyclic ring containing phosphorus:synthesis of 4-Chloro-1,4-dihydro-2 H-naphth-[2,1-c][1,2] oxaphosphorin. Ind J Chem 16B:638–645
Turan-Zitouni G, Chevallet P, Kilic FS, Erol K (2000) Synthesis of some thiazolyl-pyrazoline derivatives and preliminary investigation of their hypotensive activity. Eur J Med Chem 35:635–641
Erhan R, Mutlu A, Tayfun U, Dilek E (2001) Synthesis and antidepressant activities of some 3,5-diphenyl-2-pyrazolines. Eur J Med Chem 36:539–543
Husain MI, Shukla S (1986) Synthesis & biological activity of 4-(3-Aryl-4-exo-2-thioxothia-zolidin-5-ylimino)-3-methyl-1-(N,N-disubstituted amino-methyl)pyrazolin-5-ones. Ind J Chem 25B:983–985
Orrego-Hernández J, Portilla J (2017) Synthesis of dicyanovinyl-substituted 1-(2-pyridyl) pyrazoles: design of a fluorescent chemosensor for selective recognition of cyanide. J Org Chem 82(24):13376–13385
Ibnaouf KH, Elzupir AO, Ibrahem MA, Ali MKM, Al-Muhanna MK (2019) Optical characteristics and structural properties of 3-(p-Nitrophenyl)-5-phenyl-1H-pyrazole. J Electron Mater 48(2):861–866
Maliyappa MR, Keshavayya J, Mahanthappa M, Shivaraj Y, Basavarajappa KV (2020) 6-substituted benzothiazole based dispersed azo dyes having pyrazole moiety: synthesis, characterization, electrochemical and DFT studies. J Mol Struct 1199:126959
Orrego-Hernandez J, Portilla J (2017) Synthesis of Dicyanovinyl-substituted 1-(2-Pyridyl)pyrazoles: Design of a Fluorescent Chemosensor for selective recognition of cyanide. J Org Chem 82:13376–13385
Wang CC, Zhang D, Huang XY, Ding P, Wang Z, Zhao Y, Ye Y (2014) A fluorescence ratiometric chemosensor for Fe3+ based on TBET and its application in living cells. Talanta 128:69–74
Jin XD, Wang SF, Yin WZ, Xu T, Jiang Y, Liao Q, Xia X, Liu J (2017) A highly sensitive and selective fluorescence chemosensor for Fe3+ based on rhodamine and its application in vivo imagine. Sensors Actuators B 247:461–468
Bozkurt E, Arik M, Onagner Y (2015) A novel system for Fe3+ ion detection based on fluorescence resonance energy transfer. Sensors Actuators B 221:136–147
Zhao B, Liu T, Fang T et al (2017) A new selective chemosensors based on phenanthro[9,10-d] imidazole-coumarin with sequential“on-off-on”fluorescence response to Fe3+ and phosphate anions and its application in living cells. Sensors Actuators B 246:370–379
Li P, Zhang M, Sun XK, Guan S, Zhang G, Baumgarten M, Müllen K (2016) A dendrimer-based highly sensitive and selective fluorescence-quenching sensor for Fe3+ both in solution and as film. Biosens Bioelectron 85:785–791
Sen S, Sarkar S, Chattopadhyay B, Moirangthem A, Basu A, Dhara K, Chattopadhyay P (2012) A ratiometric fluorescence chemosensor for iron: discrimination of Fe2+and Fe3+ and living cell application. Analyst 137:3335–3342
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behaviour. J Phys Rev A 38:3098–3000
Becke AD (1993) Density-functional thermochemistry III the role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a function of the electron density. Phys Rev B 37:785–789
Zhang TT, Wang FW, Li MM, Liu JT, Miao JY, Zhao BX (2013) A simple pyrazoline-based fluorescent probe for Zn2+ in aqueous solution and imaging in living neuron cells. Sensors Actuators B 186:755–760
Kasirajan G, Krishnaswamy V, Raju N, Mahalingam M, Sadasivam M, Palathurai Subramaniam M, Ramasamy S (2017) New pyrazolo-quinoline scaffold as a reversible colorimetric fluorescent probe for selective detection of Zn2+ ions and its imaging in live cells. Journal of Photochemistry and Photobiology A 341:136–145
Li MM, Wang FW, Wang XY, Zhang TT, Xu Y, Xiao Y, Miao JY, Zhao BX (2014) A new turn-on fluorescence probe for Zn2+ in aqueous solution and imaging application in living cells. Anal Chim Acta 826:77–83
Mikata Y, Yamashita A, Kawamura A et al (2009) Bisquinoline-based fluorescent zinc sensors. Dalton Trans 19:3800–3806
Maity D, Govindaraju T (2011) Naphthaldehyde-urea/thiourea conjugates as turn-on fluorescent probes for Al3+ based on restricted C=N isomerization. Eur J Inorg Chem 36:5479–5485
Velmurugan K, Raman A, Easwaramoorthi S, Nandhakumar R (2014) Pyrene pyridine-conjugate as Ag selective fluorescent chemosensor. RSC Adv 4:35284–35289
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 462 kb)
Rights and permissions
About this article
Cite this article
Zhang, YP., Li, XF., Yang, YS. et al. A Novel Fluorescent Probe Based on Pyrazole-Pyrazoline for Fe (III) Ions Recognition. J Fluoresc 31, 29–38 (2021). https://doi.org/10.1007/s10895-020-02632-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-020-02632-w