Abstract
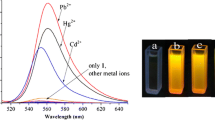
A rhodamine B-based fluorescence probe (1) for the sensitive and selective detection of Cu2+ ion has been designed and synthesized using pyridine moiety. The optical properties of this compound have been investigated in acetonitrile-water binary solution (7:3 v/v). Compound 1 is found to be an excellent sensor for a biologically/physiologically very important transition metal ion (Cu2+) using only the two very different modes of measurements (absorption and emission); one case displayed intensity enhancement whereas in other case showed intensity depletion (quenching). A mechanistic investigation has been performed to explore the static nature of quenching process. The sensor has been found to be very effective in sensing Cu2+ ion inside living cells also.




Similar content being viewed by others
References
Wu J, Liu W, Ge J, Zhang H, Wang P (2011) New sensing mechanisms for design of fluorescent chemosensors emerging in recent years. Chem Soc Rev 40(7):3483–3495
Lohani CR, Kim JM, Chung SY, Yoon J, Lee KH (2010) Colorimetric and fluorescent sensing of pyrophosphate in 100 % aqueous solution by a system comprised of rhodamine B compound and Al3+ complex. Analyst 135(8):2079–2084
de Silva AP, Gunaratne HQN, Gunnlaugsson T, Huxley AJM, McCoy CP, Rademacher JT, Rice TE (1997) Signaling recognition events with fluorescent sensors and switches. Chem Rev 97(5):1515–1566
Pu L (2004) Fluorescence of organic molecules in chiral recognition. Chem Rev 104(3):1687–1716
Gokel GW, Leevy WM, Weber ME (2004) Crown ethers: sensors for ions and molecular scaffolds for materials and biological models. Chem Rev 104(5):2723–2750
Sahana A, Banerjee A, Das S, Lohar S, Karak D, Sarkar B, Mukhopadhyay SK, Mukherjee AK, Das D (2011) A naphthalene-based Al3+ selective fluorescent sensor for living cell imaging. Org Biomol Chem 9(15):5523–5529
Prodi L, Bolletta F, Montalti M, Zaccheroni N (2000) Luminescent chemosensors for transition metal ions. Coord Chem Rev 205(1):59–83
Amendola V, Fabbrizzi L, Forti F, Licchelli M, Mangano C, Pallavicini P, Poggi A, Sacchi D, Taglieti A (2006) Light-emitting molecular devices based on transition metals. Coord Chem Rev 250(3–4):273–293
Valeur B, Leray I (2000) Design principles of fluorescent molecular sensors for cation recognition. Coord Chem Rev 205(1):3–40
High B, Bruce D, Richter MM (2001) Determining copper ions in water using electrochemiluminescence. Anal Chim Acta 449(1–2):17–22
Tapia L, Suazo M, Hodar C, Cambiazo V, Gonzalez M (2003) Copper exposure modifies the content and distribution of trace metals in mammalian cultured cells. Biometals 16(1):169–174
Sigel H (1981) Metal ions in biological systems, properties of copper, vol 12. Dekker, New York
Waggoner DJ, Bartnikas TB, Gitlin JD (1999) The role of copper in neurodeganerative disease. Neurobiol Dis 6(4):221–230
Krämer R (1998) Fluorescent chemosensors for Cu2+ ions: fast, selective, and highly sensitive. Angew Chem Int Ed 37(6):772–773
Ghosh P, Bharadwaj PK, Mandal S, Sanjib G (1996) Ni(II), Cu(II), and Zn(II) cryptate-enhanced fluorescence of a trianthrylcryptand: a potential molecular photonic OR operator. J Am Chem Soc 118(6):1553–1554
Zheng Y, Orbulescu J, Ji X, Andreopoulos FM, Pham SM, Leblanc RM (2003) Development of fluorescent film sensors for the detection of divalent copper. J Am Chem Soc 125(9):2680–2686
Royzen M, Dai Z, Canary JW (2005) Ratiometric displacement approach to Cu(II) sensing by fluorescence. J Am Chem Soc 127(6):1612–1613
Sumner JP, Westerberg NM, Stoddard AK, Hurst TK, Cramer M, Thompson RB, Fierke CA, Kopelman R (2006) DsRed as a highly sensitive, selective, and reversible fluorescence-based biosensor for both Cu+ and Cu2+ ions. Biosens Bioelectron 21(7):1302–1308
Yang H, Liu ZQ, Zhou ZG, Shi EX, Li FY, Du YK, Yi T, Huang CH (2006) Highly selective ratiometric fluorescent sensor for Cu(II) with two urea groups. Tetrahedron Lett 47(17):2911–2914
Zheng Y, Cao X, Orbulescu J, Konka V, Andreopoulos FM, Pham SM, Leblanc RM (2003) Peptidyl fluorescent chemosensors for the detection of divalent copper. Anal Chem 75(7):1706–1712
Mokhir A, Krämer R (2005) Double discrimination by binding and reactivity in fluorescent metal ion detection. Chem Commun 2244–2246
Kovács J, Rödler T, Mokhir A (2006) Chemodosimeter for CuII detection based on cyclic peptide nucleic acids. Angew Chem Int Ed 45(46):7815–7817
Dujols V, Ford F, Czarnik AW (1997) A long-wavelength fluorescent chemodosimeter selective for Cu(II) ion in water. J Am Chem Soc 119(31):7386–7387
Yang YK, Yook KJ, Tae J (2005) A rhodamine-based fluorescent and colorimetric chemodosimeter for the rapid detection of Hg2+ ions in aqueous media. J Am Chem Soc 127(48):16760–16761
Kwon JY, Jang YJ, Lee YJ, Kim KM, Seo MS, Nam W, Yoon J (2005) A highly selective fluorescent chemosensor for Pb2+. J Am Chem Soc 127(28):10107–10111
Xiang Y, Mei L, Li N, Tong A (2007) Sensitive and selective spectrofluorimetric determination of chromium(VI) in water by fluorescence enhancement. Anal Chim Acta 581(1):132–136
Nguyen T, Francis MB (2003) Practical synthetic route to functionalized rhodamine dyes. Org Lett 5(18):3245–3248
Xiang Y, Tong A, Jin P, Ju Y (2006) New fluorescent rhodamine hydrazone chemosensor for Cu(II) with high selectivity and sensitivity. Org Lett 8(13):2863–2866
Sikdar A, Panja SS, Biswas P, Roy S (2012) A rhodamine-based dual chemosensor for Cu(II) and Fe(III). J Fluoresc 22(1):443–450
Sarkar M, Banthia S, Patil A, Ansari MB, Samanta A (2006) pH-Regulated “Off–On” fluorescence signalling of d-block metal ions in aqueous media and realization of molecular IMP logic function. New J Chem 30(11):1557–1560
Yuan L, Lin W, Fung Y (2011) A rational approach to tuning the pK a values of rhodamines for living cell fluorescence imaging. Org Biomol Chem 9(6):1723–1726
Xiang Y, Li Z, Chen X, Tong A (2008) Highly sensitive and selective optical chemosensor for determination of Cu2+ in aqueous solution. Talanta 74(5):1148–1153
Ding J, Yuan L, Gao L, Chen J (2012) Fluorescence quenching of a rhodamine derivative: selectively sensing Cu2+ in acidic aqueous media. J Lumin 132(8):1987–1993
Jung HS, Kwon PS, Lee JW, Kim JI, Hong CS, Kim JW, Yan S, Lee JY, Lee JH, Joo T, Kim JS (2009) Coumarin-derived Cu2+-selective fluorescence sensor: synthesis, mechanisms, and applications in living cells. J Am Chem Soc 131(5):2008–2012
Ressalan S, Iyer CSP (2005) Absorption and fluorescence spectroscopy of 3-hydroxy-3-phenyl-1-o-carboxyphenyltriazene and its copper (II), nickel (II) and zinc (II) complexes: a novel fluorescence sensor. J Lumin 111(3):121–129
Liu J, Li C, Li F (2011) Fluorescence turn-on chemodosimeter-functionalized mesoporous silica nanoparticles and their application in cell imaging. J Mater Chem 21(20):7175–7181
Banerjee A, Sahana A, Das S, Lohar S, Guha S, Sarkar B, Mukhopadhyay SK, Mukherjee AK, Das D (2012) A naphthalene exciplex based Al3+ selective on-type fluorescent probe for living cells at the physiological pH range: experimental and computational studies. Analyst 137(9):2166–2175
Liu WY, Li HY, Zhao BX, Miao JY (2012) A new fluorescent and colorimetric probe for Cu2+ in live cells. Analyst 137(15):3466–3469
Acknowledgments
This work was fully supported by DST, Govt. of India (No. SR/FT/CS-047/2008). The authors are also thankful to Dr. A. K. Patra and Dr. D. Sukul for fruitful discussion. We gratefully acknowledge the help of Dr. Anjil Srivastava and Minati Behra (Dept. of Biotechnology NIT Durgapur) for fluorescence image recording.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 1682 kb)
Rights and permissions
About this article
Cite this article
Sikdar, A., Roy, S., Haldar, K. et al. Rhodamine-Based Cu2+ -Selective Fluorosensor: Synthesis, Mechanism, and Application in Living Cells. J Fluoresc 23, 495–501 (2013). https://doi.org/10.1007/s10895-013-1169-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-013-1169-y