Abstract
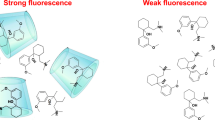
The thermodynamics of the dimer formation of 2I,3I-O-(o-xylylene)-per-O-Me-γ-cyclodextrin (XmγCD) in aqueous solution was studied by fluorescence techniques, Molecular Mechanics and Molecular Dynamics. Lifetime averages \( \left\langle \tau \right\rangle \), obtained from fluorescence decay profiles upon excitation of the xylylene appended group, were used as the property sensitive to the association process. The dimerization equilibrium constants (K D) were obtained from non-linear regression analysis of the plots of \( \left\langle \tau \right\rangle \) against [XmγCD] at several temperatures and they were compared with the values obtained for the counterparts Xmα- and XmβCDs. The van’t Hoff plot allows us to obtain the ΔH and ΔS showing that the dimerization process was also entropically disfavoured. Molecular Mechanics as well as Molecular Dynamics calculations in the presence of water were also employed to study the conformational behaviour of isolated XmγCDs, the possible structure of the dimers formed and the driving forces involved in such association processes. Results indicate that those conformations where Xy moiety does not block the cavity entrance are favoured. Dimers are preferably formed by head-to-head CD approaching. However, the formation of stable head-to-tail is not dismissed.












Similar content being viewed by others
References
Szejtli J, Osa T (1996) Comprehensive supramolecular chemistry, Vol. 3. Pergamon, Oxford
D’Souza VT, Lipkowitz KB (1998) Cyclodextrins: introduction. Chem Rev 98(5):1741–1742. doi:10.1021/cr980027p
Harada A (2001) Cyclodextrin-based molecular machines A. Acc Chem Res 34(6):456–464. doi:10.1021/ar000174l
Nepogodiev SA, Stoddart JF (1998) Cyclodextrin-based catenanes and rotaxanes. Chem Rev 98(5):1959–1976. doi:10.1021/cr970049w
Madrid JM et al (1999) Experimental thermodynamics and molecular mechanics calculations of inclusion complexes of 9-methyl anthracenoate and 1-methyl pyrenoate with β-cyclodextrin. J Phys Chem B 103(23):4847–4853. doi:10.1021/jp9838240
Cervero M, Mendicuti F (2000) Inclusion complexes of dimethyl 2, 6-naphthalenedicarboxylate with α- and β-cyclodextrins in aqueous medium: thermodynamics and molecular mechanics studies. J Phys Chem B 104(7):1572–1580. doi:10.1021/jp993418w
Di Marino A, Mendicuti F (2002) Fluorescence of the complexes of 2-methylnaphthoate and 2-hydroxypropyl-α-, -β-, and -γ-cyclodextrins in aqueous solution. Appl Spectrosc 56(12):1579–1587. doi:10.1366/000370202321115841
Pastor I, Di Marino A, Mendicuti F (2005) Complexes of dihexyl 2, 6-naphthalenedicarboxylate with α- and β-cyclodextrins: fluorescence and molecular modelling. J Photochem Photobiol 173(3):238–247. doi:10.1016/j.jphotochem.2005.04.003
Di Marino A, Rubio L, Mendicuti F (2007) Fluorescence and molecular mechanics of 1-methyl naphthalenecarboxylate/cyclodextrin complexes in aqueous medium. J Incl Phenom Macrocycl Chem 58(1–2):103–114. doi:10.1007/s10847-006-9129-7
Alvariza C et al (2007) Binding of dimethyl 2,3-naphthalenedicarboxylate with α-, β- and γ-cyclodextrins in aqueous solution. Spectroc Acta Pt A Mol Biomolec Spectr 67A(2):420. doi:10.1016/j.saa.2006.07.039
Kodaka M, Fukaya T (1989) Induced circular dichroism spectrum of a α-cyclodextrin complex with heptylviologen. Bull Chem Soc Jpn 62(4):1154–1157. doi:10.1246/bcsj.62.1154
Ueno A et al (1990) Host-guest sensory system of dansyl-modified β-cyclodextrin for detecting steroidal compounds by dansyl fluorescence. Chem Lett 4:605–608. doi:10.1246/cl.1990.605
Berberan-Santos MN et al (1992) Multichromophoric cyclodextrins.1. Synthesis of o-naphthoyl-β-cyclodextrins and investigation of excimer formation and energy hopping. J Am Chem Soc 114(16):6427–6436. doi:10.1021/ja00042a021
Berberan-Santos MN et al (1993) Multichromophoric cyclodextrins.2. Inhomogeneous spectral broadening and directed energy hopping. J Phys Chem 97(44):11376–11379. doi:10.1021/j100146a006
Kodaka M (1993) A general rule for circular dichroism induced by a chiral macrocycle. J Am Chem Soc 115(9):3702–3705. doi:10.1021/ja00062a040
Hamasaki K et al (1993) Fluorescent sensors of molecular recognition. Modified cyclodextrins capable of exhibiting guest-responsive twisted intramolecular charge transfer fluorescence. J Am Chem Soc 115(12):5035–5040. doi:10.1021/ja00065a012
Gravett DM, Guillet JE (1993) Synthesis and photophysics of a water-soluble, naphthalene-containing β-cyclodextrin. J Am Chem Soc 115(14):5970–5974. doi:10.1021/ja00067a011
Jullien L et al (1994) Antenna effect in multichromophoric cyclodextrins. Angew Chem Int Ed Engl 106(23/24):2582–2584
Wang Y et al (1994) Dansyl-β-cyclodextrins as fluorescent sensors responsive to organic compounds. Bull Chem Soc Jpn 67(6):1598–1607. doi:10.1246/bcsj.67.1598
McAlpine SR, Garcia-Garibay MA (1996) Inside–outside isomerism of β-cyclodextrin covalently linked with a naphthyl group. J Am Chem Soc 118(11):2750–2751. doi:10.1021/ja953061j
Park JW, Choi NH, Kim JH (1996) Facile dimerization of viologen radical cations covalently bonded to β-cyclodextrin and suppression of the dimerization by β-cyclodextrin and amphiphiles. J Phys Chem 100(2):769–774. doi:10.1021/jp9520308
Ikeda H (1996) Fluorescent cyclodextrins for molecule sensing: fluorescent properties, NMR characterization, and inclusion phenomena of N-dansylleucine-modified cyclodextrins. J Am Chem Soc 118(45):10980–10988. doi:10.1021/ja960183i
McAlpine SR, García-Garibay MA (1996) Binding studies of adamantanecarboxylic acid and a naphthyl-bound β-cyclodextrin by variable temperature 1H NMR. J Org Chem 61(23):8307–8309. doi:10.1021/jo9613794
Ikeda H et al (1997) NMR studies of conformations of N-dansyl-L-leucine-appended and N-dansyl-D-leucine-appended β-cyclodextrin as fluorescent indicators for molecular recognition. J Org Chem 62(5):1411–1418. doi:10.1021/jo960425x
McAlpine SR, Garcia-Garibay MA (1998) Studies of naphthyl-substituted β-cyclodextrins. Self-aggregation and inclusion of external guests. J Am Chem Soc 120(18):4269–4275. doi:10.1021/ja972810p
Schneider H-J, Hacket F, Rüdiger V (1998) NMR studies of cyclodextrins and cyclodextrin complexes. Chem Rev 98(5):1755–1785. doi:10.1021/cr970019t
Ishizu T, Kintsu K, Yamamoto H (1999) NMR study of the solution structures of the inclusion complexes of β-cyclodextrin with (+)-catechin and (−)-epicatechin. J Phys Chem B 103(42):8992–8997. doi:10.1021/jp991178e
Ikunaga T et al (1999) The effects of avidin on inclusion phenomena and fluorescent properties of biotin-appended dansyl-modified β-cyclodextrin. Chem Eur J 5(9):2698–2704. doi:10.1002/(SICI)1521-3765(19990903)5:9<2698::AID-CHEM2698>3.0.CO;2-G
Berberan-Santos MN et al (1999a) Multichromophoric cyclodextrins.6. Investigation of excitation energy hopping by Monte-Carlo simulations and time-resolved fluorescence anisotropy. J Am Chem Soc 121(11):2526–2533. doi:10.1021/ja983601n
Berberan-Santos MN et al (1999b) Multichromophoric cyclodextrins. 8. Dynamics of homo- and heterotransfer of excitation energy in inclusion complexes with fluorescent dyes. J Am Chem Soc 122(48):11876–11886. doi:10.1021/ja000995l
Hoshino T et al (2000) Daisy chain necklace: tri[2]rotaxane containing cyclodextrins. J Am Chem Soc 122(40):9876–9877. doi:10.1021/ja0018264
Fujimoto T et al (2000) The first lipophilic face-to-face dimers of permethylated α-cyclodextrin-azobenzene dyads through a p-xylylene spacer. Chem Lett 5:564–565. doi:10.1246/cl.2000.564
Fujimoto T (2000) The first lipophilic face-to-face dimers of permethylated α-cyclodextrin-azobenzene dyads through a p-xylylene spacer. Chem Lett 7:764–765. doi:10.1246/cl.2000.764
Mirzoian A, Kaifer AE (1999) Electrochemically controlled self-complexation of cyclodextrin-viologen conjugates. Chem Commun (Camb) 16:1603–1604. doi:10.1039/a904095a
Liu Y et al (2000) Molecular Interpenetration within the columnar structure of crystalline anilino-β-cyclodextrin. Org Lett 2:2761–2763. doi:10.1021/ol000135+
Inoue Y et al (2000) Supramolecular photochirogenesis.2.Enantio-differentiating photoisomerization of cyclooctene included and sensitized by 6-O-modified cyclodextrins. J Org Chem 65(23):8041–8050. doi:10.1021/jo001262m
Fujimoto T et al (2001) Photoswitching of the association of a permethylated α-cyclodextrin-azobenzene dyad forming a Janus [2]pseudorotaxane. Tetrahedron Lett 42(45):7987–7989. doi:10.1016/S0040-4039(01)01563-5
Aoyagi T et al (2001) Fluorescence properties, induced-fit guest binding and molecular recognition abilities of modified γ-cyclodextrins bearing two pyrene moieties. Bull Chem Soc Jpn 74(1):157–164. doi:10.1246/bcsj.74.157
Park K (2001) Preparation and self-inclusion properties of p-xylylenediamine-modified β-cyclodextrins: dependence on the side of modification. J Chem Soc Perkin Trans 2(11):2114–2118. doi:10.1039/b105388b
Kuwabara T et al (2002) Hetero-dimerization of dye-modified cyclodextrins with native cyclodextrins. J Org Chem 67(3):720–725. doi:10.1021/jo010696u
Park JW et al (2002) Facile dimerization and circular dichroism characteristics of 6-O-(2-Sulfonato-6-naphthyl)-β-cyclodextrin. J Phys Chem B 106(20):5177–5183. doi:10.1021/jp014191j
Park JW et al (2002) Face selectivity of inclusion complexation of viologens with β-cyclodextrin and 6-O-(2-sulfonato-6-naphthyl)-β-cyclodextrin. J Phys Chem B 106(29):7186–7192. doi:10.1021/jp020428f
Harada A et al (2003) Supramolecular polymers formed by cinnamoyl cyclodextrins. J Polym Sci A Polymer Chem (Kyoto) 41(22):3519–3523
Liu Y et al (2003) Supra-molecular self-assemblies of β-cyclodextrins with aromatic tethers: factors governing the helical columnar versus linear channel super-structures. Org Chem 68(22):8345–8352. doi:10.1021/jo034632q
Park JW et al (2003) Homo-dimerization and hetero-association of 6-O-(2-sulfonato-6-naphthyl)-γ-cyclodextrin and 6-deoxy-(pyrene-1-carboxamido)-β-cyclodextrin. J Org Chem 68(18):7071–7076. doi:10.1021/jo034623h
Liu Y et al (2003) Binding ability and self-assembly behavior of linear polymeric supramolecules formed by modified β-cyclodextrin. Org Lett 5(3):251–254. doi:10.1021/ol027146i
Balzani V (2003) Molecular devices and machines: a journey into the Nanoworld. Wiley-VCH, Weinhein
Miyauchi M et al (2005) A [2]Rotaxane capped by a cyclodextrin and a guest: formation of supramolecular [2]Rotaxane polymer. J Am Chem Soc 127(7):2034–2035. doi:10.1021/ja042840+
Miyauchi M, Harada A (2005) A helical supramolecular polymer formed by host–guest interactions. Chem Lett 34(1):104–105. doi:10.1246/cl.2005.104
Miyauchi M et al (2005) Chiral supramolecular polymers formed by host–guest interactions. J Am Chem Soc 127(9):2984–2989. doi:10.1021/ja043289j
Hasewaga Y et al (2005) Supramolecular polymers formed from βciclodextrins dimer linked by poly(ethyleneglycol) and guest dimers. Macromolecules 38(9):3724–3730. doi:10.1021/ma047451e
Park JW (2005) Efficient inclusion complexation and intra-complex excitation energy transfer between aromatic group-modified β-cyclodextrins and a hemicyanine dye. J Photochem Photobiol Chem 173(3):271–278. doi:10.1016/j.jphotochem.2005.04.006
Ikeda H et al (2005) Skeleton-selective fluorescent chemosensor based on cyclodextrin bearing a 4-amino-7-nitrobenz-2-oxa-1, 3-diazole moiety. Org Biomol Chem 3(23):4262–4267. doi:10.1039/b508477f
Nakashima H, Yoshida N (2006) Fluorescent detection for cyclic and acyclic alcohol guests by naphthalene-appended amino-β-cyclodextrins. Org Lett 8(22):4997–5000. doi:10.1021/ol0616079
Wu A et al (2006) Investigation on photophysical properties of a substituted 3H-indole-modified β-cyclodextrin. J Photochem Photobiol Chem 182(2):174–180. doi:10.1016/j.jphotochem.2006.02.008
González-Álvarez MJ et al (2008) Study of the conformational and self-aggregation properties of 2I, 3I-O-(o-Xylylene)-per-O-Me-α- and -β-cyclodextrins by fluorescence and molecular modeling. J Chem Phys 112:13717–13729
Engeldinger E et al (2003) Capped cyclodextrins. Chem Rev 103(11):4147–4173. doi:10.1021/cr030670y
Balbuena P et al (2007) One-pot regioselective synthesis of 2I,3I-O-(o-xylylene)-capped cyclomaltooligosaccharides: tailoring the topology and supramolecular properties of cyclodextrins. Chem Commun (Camb) 31:3270–3272 doi:10.1039/b705644c
Wang W (1997) Synthetic study of selective benzylic oxidation. J Org Chem 62(19):6598–6602. doi:10.1021/jo962172d
Uekama K, Irie T (1987) Cyclodextrins and their industrial uses. D.Duchême Edition Sante, Paris, Chapter 10
Aree T et al (2000) Novel type of thermostable channel clathrate hydrate formed by heptakis(2, 6-di-O-methyl)-β-cyclodextrin·15H2O-a paradigm of the hydrophobic effect. Angew Chem Int Ed 39(5):897–899. doi:10.1002/(SICI)1521-3773(20000303)39:5<897::AID-ANIE897>3.0.CO;2-R
Jefrey GA (1996) In: Atwood JL, Davies JED, MacNicol DD, Vögtle F (eds) Comprehensive supramolecular chemistry, Vol. 6, Chap. 23. Pergamon Press, Oxford
Starikov EB et al (2001) Negative solubility coefficient of methylated cyclodextrins in water: a theoretical study. Chem Phys Lett 336:504–510. doi:10.1016/S0009-2614(01)00160-9
Lakowicz JR (1999a) Principles of fluorescence spectroscopy, 2nd edn. Kluwer, New York, p 298
Lakowicz JR (1999b) Principles of fluorescence spectroscopy, 2nd edn. Kluwer, New York, p 239
SYBYL molecular modeling software (Version 6.9), Tripos Associates, Inc., St. Louis, MO
Clark M et al (1989) Validation of the general purpose Tripos 5.2 force field. J Comput Chem 10(8):982–1012. doi:10.1002/jcc.540100804
Brunel Y et al (1975) Program of minimization of the empirical energy of a molecule by a simple method. Tetrahedron 31(8):1075–1091. doi:10.1016/0040-4020(75)80129-3
Press W et al (1988) Numerical recipes: the art of scientific computing. Cambridge University Press, Cambridge, p 312
Frisch MJ et al (2004) MOPAC (AM1): included in the Gaussian 03 package. Gaussian 03, revision C.02. Gaussian, Wallingford, CT
Blanco M (1991) Molecular silverware. I. General solutions to excluded volume constrained problems. J Comput Chem 12(2):237–247. doi:10.1002/jcc.540120214
Pozuelo J et al (1996) Inclusion complexes of chain molecules with cycloamyloses. 1. Conformational analysis of the isolated cycloamyloses using molecular dynamics simulations. Comput Theor Polym Sci 6:125–134
Acknowledgments
This work was supported by the Spanish MEC (projects CTQ2006-15515C02-01/BQU, CTQ2007-61180/PPQ, CTQ2005-04710/BQU and CTQ2008-03149/BQU), the Junta de Andalucía (P06-FQM-01601), the Comunidad de Madrid (S-055/MAT/0227) and the Junta de Castilla-La Mancha (grant to M.J.G-A). FM and MJG-A acknowledge the assistance of M. L. Heijnen with the preparation of the manuscript and L. M. Frutos for his valuable co-operation in some calculations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
González-Álvarez, M.J., Vicente, J., Mellet, C.O. et al. Thermodynamics of the Dimer Formation of 2I,3I-O-(o-Xylylene)-per-O-Me-γ-cyclodextrin: Fluorescence, Molecular Mechanics and Molecular Dynamics. J Fluoresc 19, 975–988 (2009). https://doi.org/10.1007/s10895-009-0497-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-009-0497-4