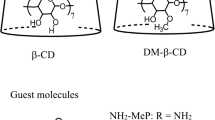
Abstract
Fluorescence self-quenching occurs at high concentration. Inhibition of self-quenching by inclusion of fluorescence emitters inside the hydrophobic cavity of β-cyclodextrin (β-CD) has been addressed taking the example of the fluorescence behavior of Tramadol hydrochloride. Indeed complexation by β-CD enhanced fluorescence emission of Tramadol under conditions where self-quenching was operative. A quantitative account of self-quenching and its inhibition by β-CD was done through determination of complexation equilibrium by 1H NMR experiments and a detailed study of absorption and fluorescence properties. Tramadol and β-CD associate as a complex of 1:1 stoichiometry with a formation constant K11 = 260. Complexation of Tramadol by β-CD does not cause modification of its absorbance and fluorescence spectra. Fluorescence self-quenching of Tramadol above ∼ 1 mmol·L−1 was characterized by a Stern–Volmer constant K = 810 L·mol−1. Inhibition of self-quenching by formation of an inclusion complex was manifested by lower Stern–Volmer constants in the presence of β-CD. Such study required a correct account of Inner Filter Effects on fluorescence, which is mandatory in all physicochemical studies using fluorescence where concentrations are rather high.
Graphical abstract











Similar content being viewed by others
References
Frankewich, R.P., Thimmaiah, K.N., Hinze, W.L.: Evaluation of the relative effectiveness of different water-soluble β-cyclodextrin media to function as fluorescence enhancement agents. Anal. Chem. 63, 2924–2933 (1991)
Bortolus, P., Monti, S.: Photochemistry in cyclodextrin cavities. Adv. Photochem. 21, 1–133 (1996)
Ramamurthy, V., Eaton, D.F.: Photochemistry and photophysics within cyclodextrin cavities. Acc. Chem. Res. 21, 300–306 (1988)
Chen, J., Tang, B.Z.: Restricted intramolecular rotations: a mechanism for aggregation-induced emission. In: Qin, A., Tang, B.Z. Aggregation-Induced Emission, pp. 307–322. Wiley, Chichester (2014)
Hwang, H., Kim, H., Myong, S.: Protein induced fluorescence enhancement as a single molecule assay with short distance sensitivity. Proc. Natl Acad. Sci. USA 108, 7414–7418 (2011)
Peccati, F., Hernando, J., Blancafort, L., Solans-Monfort, X., Sodupe, M.: Disaggregation-induced fluorescence enhancement of NIAD-4 for the optical imaging of amyloid-β fibrils. Phys. Chem. Chem. Phys. 17, 19718–19725 (2015)
Xu, J.-L., Quan, Y., Li, Q.-Y., Lu, H., Wu, H., Yin, J., Wang, X.-J., Zhang, Q.: Significant emission enhancement of a bolaamphiphile with salicylaldehyde azine moiety induced by the formation of [2]pseudorotaxane with γ-cyclodextrin. RSC Adv. 5, 88176–88180 (2015)
Deng, S.-L., Huang, P.-C., Lin, L.-Y., Yang, D.-J., Hong, J.-L.: Complex from ionic β-cyclodextrin polyrotaxane and sodium tetraphenylthiophenesulfonate: restricted molecular rotation and aggregation-enhanced emission. RSC Adv. 5, 19512–19519 (2015)
Sbai, M., Lyazidi, S.A., Lerner, D.A., del Castillo, B., Martin, M.A.: Modified β-cyclodextrins as enhancers of fluorescence emission of carbazole alkaloid derivatives. Anal. Chim. Acta 303, 47–55 (1995)
Sbai, M., Lyazidi, S.A., Lerner, D.A., del Castillo, B., Martin, M.A.: Stoichiometry and association constants of the inclusion complexes of ellipticine with modified β-cyclodextrin. Analyst 121, 1561–1564 (1996)
Shuang, S.-M., Guo, S.-Y., Li, L., Cai, M.-Y., Pan, J.-H.: β-Cyclodextrin derivatives as fluorescence enhancers of the drug, hesperidin. Anal. Lett. 31, 1357–1366 (1998)
Galian, R.E., Veglia, A.V.: Fluorescence quenching inhibition of substituted indoles by neutral and ionized cyclodextrins nanocavities. J. Photochem. Photobiol. A 187, 356–362 (2007)
Oddy, F.E., Brovelli, S., Stone, M.T., Klotz, E.J.F., Cacialli, F., Anderson, H.L.: Influence of cyclodextrin size on fluorescence quenching in conjugated polyrotaxanes by methyl viologen in aqueous solution. J. Mater. Chem. 19, 2846–2852 (2009)
Bracamonte, A.G., Veglia, A.V.: Cyclodextrins nanocavities effects on basic and acid fluorescence quenching of hydroxy-indoles. J. Photochem. Photobiol. A 261, 20–25 (2013)
Vazzana, M., Andreani, T., Fangueiro, J., Faggio, C., Silva, C., Santini, A., Garcia, M.L., Silva, A.M., Souto, E.B.: Tramadol hydrochloride: pharmacokinetics, pharmacodynamics, adverse side effects, co-administration of drugs and new drug delivery systems. Biomed. Pharmacother. 70, 234–238 (2015)
Grond, S., Sablotzki, A.: Clinical pharmacology of tramadol. Clin. Pharmacokinet. 43, 879–923 (2004)
Bamigbade, T.A., Davidson, C., Langford, R.M., Stamford, J.A.: Actions of tramadol, its enantiomers and principal metabolite, O-desmethyltramadol, on serotonin (5-HT) efflux and uptake in the rat dorsal raphe nucleus. Brit. J. Anaesth. 79, 352–356 (1997)
Hennies, H.H., Friderichs, E., Schneider, J.: Receptor binding, analgesic and antitussive potency of tramadol and other selected opioids. Arzneim.-Forsch. 38, 877–880 (1988)
Reimann, W., Hennies, H.-H.: Inhibition of spinal noradrenaline uptake in rats by the centrally acting analgesic tramadol. Biochem. Pharmacol. 47, 2289–2293 (1994)
Loftsson, T., Duchêne, D.: Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 329, 1–11 (2007)
Bilensoy, E. (ed.): Cyclodextrins in Pharmaceutics, Cosmetics, and Biomedicine, Current and Future Industrial Applications. Wiley, Hoboken (2011)
Stella, V.J., Rao, V.M., Zannou, E.A., Zia, V.: Mechanisms of drug release from cyclodextrin complexes. Adv. Drug Deliv. Rev. 36, 3–16 (1999)
Duchêne, D., Bochot, A., Yu, S.-C., Pépin, C., Seiller, M.: Cyclodextrins and emulsions. Int. J. Pharm. 266, 85–90 (2003)
Yu, S.-C., Bochot, A., Le Bas, G., Chéron, M., Mahuteau, J., Grossiord, J.-L., Seiller, M., Duchêne, D.: Effect of camphor/cyclodextrin complexation on the stability of O/W/O multiple emulsions. Int. J. Pharm. 261, 1–8 (2003)
Anton Smith, A., Manavalan, R., Kannan, K., Rajendiran, N.: Spectral characteristics of tramadol in different solvents and β-cyclodextrin. Spectrochim. Acta A 74, 469–477 (2009)
Box, K.J., Comer, J.E.A.: Using measured pKa, LogP and solubility to investigate supersaturation and predict BCS class. Curr. Drug Metab. 9, 869–878 (2008)
Schneider, H.-J., Hacket, F., Rüdiger, V.: NMR studies of cyclodextrins and cyclodextrin complexes. Chem. Rev. 98, 1755–1785 (1998)
Smyj, R., Wang, X.-P., Han, F.: Tramadol hydrochloride. Profil. Drug Subst. Excip. Relat. Methodol. 38, 463–494 (2013)
Wood, D.J., Hruska, F.E., Saenger, W.: 1H NMR study of the inclusion of aromatic molecules in α-cyclodextrin. J. Am. Chem. Soc. 99, 1735–1740 (1977)
Salvatierra, D., Jaime, C., Virgili, A., Sánchez-Ferrando, F.: Determination of the inclusion geometry for the β-cyclodextrin/benzoic acid complex by NMR and molecular modeling. J. Org. Chem. 61, 9578–9581 (1996)
Valeur, B., Berberan-Santos, M.N.: Molecular Fluorescence: Principles and Applications, 2nd edn., p. 69. Wiley-VCH, Weinheim (2012)
Lakowicz, J.R.: Principles of Fluorescence Spectroscopy, 3rd edn., pp. 55–56. Springer, New York (2006)
MacDonald, B.C., Lvin, S.J., Patterson, H.: Correction of fluorescence inner filter effects and the partitioning of pyrene to dissolved organic carbon. Anal. Chim. Acta 338, 155–162 (1997)
Lakowicz, J.R.: Principles of Fluorescence Spectroscopy, 3rd edn., pp. 277–284. Springer, New York (2006)
Arad-Yellin, R., Eaton, D.F.: Excited-state reactivity changes induced by complexation with cyclodextrins: Inclusion of 2,2-bis(α-naphthylmethyl)-1,3-dithiane into β- and γ-cyclodextrins. J. Phys. Chem. 87, 5051–5055 (1983)
Valeur, B., Berberan-Santos, M.N.: Molecular Fluorescence: Principles and Applications, 2nd edn., pp. 213–261. Wiley, Weinheim (2012)
Lakowicz, J.R.: Principles of Fluorescence Spectroscopy, 3rd edn., pp. 331–351. Springer, New York (2006)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zidane, S., Maiza, A., Bouleghlem, H. et al. Inclusion complex of Tramadol in β-cyclodextrin enhances fluorescence by preventing self-quenching. J Incl Phenom Macrocycl Chem 93, 253–264 (2019). https://doi.org/10.1007/s10847-018-0874-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-018-0874-1