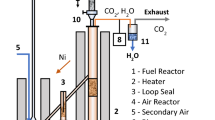
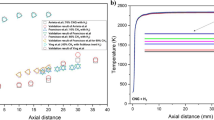
Consideration has been given to the current status of research on the development of kinetic models of combustion of kerosene and its components. Surrogate models of kerosene have been analyzed that describe the physical and chemical properties of an actual fuel and are used in developing detailed and reduced kinetic models. Experimental data have been reviewed based on which testing of the kinetic models with a varying degree of complexity is carried out. Examples of the use of kinetic models in modeling numerically processes occurring in actual power-generating units have been given.
Similar content being viewed by others
References
C. Segal, The Scramjet Engine: Processes and Characteristics, Cambridge University Press, Cambridge (2009).
M. S. Assad, O. G. Penyazkov, I. I. Chernukho, and Khaled Alhussan, Detonation regime of combustion of heptane and Jet A-1 propellant in a less than 0.5-m-long small-size combustor, J. Eng. Phys. Thermophys., 94, No. 5, 1285–1289 (2021).
E. D. Gonzalez-Juez, A. R. Kerstein, R. Ranjan, and S. Menon, Advances and challenges in modeling high-speed turbulent combustion in propulsion systems, Prog. Energy Combust. Sci., 60, No. 1, 26–67 (2017).
F. Battin-Leclerc, Detailed chemical kinetic models for the low-temperature combustion of hydrocarbons with application to gasoline and diesel fuel surrogates, Prog. Energy Combust. Sci., 34, No. 4, 440–498 (2008).
J. M. Simmie, Detailed chemical kinetic models for the combustion of hydrocarbon fuels, Prog. Energy Combust. Sci., 29, No. 6, 599–631 (2003).
C. K. Westbrook, W. J. Pitz, O. Herbinet, H. J. Curran, and E. J. Silke, A comprehensive detailed chemical kinetic reaction mechanism for combustion of n-alkane hydrocarbons from n-octane to n-hexadecane, Combust. Flame, 156, No. 1, 181–199 (2009).
M. Zeng, W. Yuan, W. Li, Y. Zhang, C. Cao, T. Li, and J. Zou, A comprehensive experimental and kinetic modeling study of n-tetradecane combustion, Energy Fuels, 31, No. 11, 12712–12720 (2017).
P. Dagaut and M. Cathonnet, The ignition, oxidation, and combustion of kerosene: A review of experimental and kinetic modeling, Prog. Energy Combust. Sci., 32, No. 1, 48–92 (2006).
A. J. Dean, O. G. Penyazkov, K. L. Sevruk, and B. Varatharajan, Autoignition of surrogate fuels at elevated temperatures and pressures, Proc. Combust. Inst., 31, No. 2, 2481–2488 (200 7).
A. A. Konn ov, A. Mohamm ad, V. R. Kisho re, N. I. K im, C. Prathap, and S. Kumar, A comprehensive review of measurements and data analysis of laminar burning velocities for various fuel + air mixtures, Prog. Energy Combust. Sci., 68, No. 1, 197–267 (2018).
P. Dagaut, F. Karsenty, G. Dayma, P. Dievart, C. Hadj-Ali, A. Mze-Ahmed, M. Braun-Unkhoff, J. Herzler, T. Kathrotia, T. Kick, C. Naumann, U. Riedel, and L. Thomas, Experimental and detailed kinetic model for the oxidation of a gas to Liquid (GtL) jet fuel, Combust. Flame, 161, No. 3, 835–847 (2014).
M. Wang, R. Dewil, K. Maniatis, J. Wheeldon, T. Tan, J. Baeyens, and Y. Fang, Biomass-derived aviation fuels: Challenges and perspective, Prog. Energy Combust. Sci., 74, No. 1, 31–49 (2019).
O. V. Matvienko, Mathematical modeling of the heat transfer and chemical reaction of a swirling flow of a dissociated gas, J. Eng. Phys. Thermophys., 89, No. 1, 127–134 (2016).
E. Garnier, N. Adams, and P. Sagaut, Large Eddy Simulation for Compressible Flows, Springer, Berlin (2009).
S. Subramaniam, Lagrangian-Eulerian methods for multiphase flows, Prog. Energy Combust. Sci., 39, No. 2, 215–245 (2013).
H. Koo, V. Raman, and P. L. Varghese, Direct numerical simulation of supersonic combustion with thermal nonequilibrium, Proc. Combust. Inst., 35, No. 2, 2145–2153 (2015).
T. Edwards, Kerosene fuels for aerospace propulsion — Composition and properties, AIAA Paper, No. 2002-3874 (2002).
W. Huang, F. Chen, H. Liu, and X. Huang, Modeling chemical mechanism for surrogate jet fuel under scramjet operating conditions, AIAA Paper, No. 2016-0182 (2016).
G. Ya. Gerasimov and S. A. Losev, Kinetic models of combustion of kerosene and its components, J. Eng. Phys. Thermophys., 78, No. 6, 1059–1070 (2005).
N. Zettervall, C. Fureby, and E. J. K. Nilsson, A reduced chemical kinetic reaction mechanism for kerosene–air combustion, Fuel, 269, Article ID 117446, 1–11 (2020).
P. Dagaut, On the kinetics of hydrocarbons oxidation from natural gas to kerosene and diesel fuel, Phys. Chem. Chem. Phys., 4, No. 11, 2079–2094 (2002).
S. Granata, T. Faravelli, and E. Ranzi, A wide range kinetic modeling study of the pyrolysis and combustion of naphthenes, Combust. Flame, 132, No. 4, 533–544 (2003).
N. A. Slavinskaya, A. Zizin, and M. Aigner, On model design of a surrogate fuel formulation, J. Eng. Gas Turbines Power, 132, No. 11, Article ID 111501 (2010).
E. Song and J. Song, Modeling of kerosene combustion under fuel-rich conditions, Adv. Mech. Eng., 9, No. 7, 1–11 (2017).
S. Dooley, S. H. Won, M. Chaos, J. Heyne, Y. Ju, F. L. Dryer, K. Kumar, C. J. Sung, H. Wang, M. A. Oehlschlaeger, R. J. Santoro, and T. A. Litzinger, A jet fuel surrogate formulated by real fuel properties, Combust. Flame, 157, No. 12, 2333–2339 (2010).
D. Kim, J. Martz, and A. Violi, A surrogate for emulating the physical and chemical properties of conventional jet fuel, Combust. Flame, 161, No. 6, 1489–1498 (2014).
Y. Mao, L. Yu, Z. Wu, W. Tao, S. Wang, C. Ruan, L. Zhu, and X. Lu, Experimental and kinetic modeling study of ignition characteristics of RP-3 kerosene over low-to-high temperature ranges in a heated rapid compression machine and a heated shock tube, Combust. Flame, 203, No. 1, 157–169 (2019).
Z. Wu, Y. Mao, M. Raza, J. Zhu, Y. Feng, S. Wang, Y. Qian, L. Yu, and X. Lu, Surrogate fuels for RP-3 kerosene formulated by emulating molecular structures, functional groups, physical and chemical properties, Combust. Flame, 208, No. 1, 388–401 (2019).
N. Slavinskaya, U. Riedel, E. Saibov, J. Herzler, C. Naumann, M. Saffaripour, and L. Thomas, Kinetic surrogate model for GRL kerosene, AIAA Paper, No. 2014–0126 (2014).
N. Al-Esawi and M. Al Qubeissi, A new approach to formulation of complex fuel surrogates, Fuel, 283, Article ID 118923, 1–9 (2021).
N. S. Titova, S. A. Torokhov, and A. M. Starik, On kinetic mechanisms of oxidation of n-decane, Fiz. Goreniya Vzryva, 47, No. 2, 3–22 (2011).
A. A. Konnov, Remaining uncertainties in the kinetic mechanism of hydrogen combustion, Combust. Flame, 152, No. 4, 507–528 (2008).
Z. Hong, D. F. Davidson, and R. K. Hanson, An improved H2/O2 mechanism based on recent shock tube/laser absorption measurements, Combust. Flame, 158, No. 4, 633–644 (2011).
G. Ya. Gerasimov and O. P. Shatalov, Kinetic mechanism of combustion of hydrogen–oxygen mixtures, J. Eng. Phys. Thermophys., 86, No. 5, 987–995 (2013).
N. A. Slavinskaya, M. Abbasi, J. H. Starcke, R. Whitside, A. Mirzayeva, U. Riedel, W. Li, J. Oreluk, A. Hegde, A. Packard, M. Frenklach, G. Gerasimov, and O. Shatalov, Development of an uncertainty quantification predictive chemical reaction model for syngas combustion, Energy Fuels, 31, No. 3, 2274–2297 (2017).
D. L. Baulch, C. T. Bowman, C. J. Cobos, R. A. Cox, T. Just, J. A. Kerr, M. J. Pilling, D. Stocker, J. Troe, W. Tsang, R. W. Walker, and J. Warnatz, Evaluated kinetic data for combustion modeling: Supplement II, J. Phys. Chem. Ref. Data, 34, No. 3, 757–1397 (2005).
C. V. Naik and A. M. Dean, Detailed kinetic modeling of ethane oxidation, Combust. Flame, 145, No. 1, 16–37 (2006).
E. Ranzi, A. Frassoldati, A. Stagni, M. Pelucchi, A. Cuoci, and T. Faravelli, Reduced kinetic schemes of complex reaction systems: Fossil and biomass-derived transportation fuels, Int. J. Chem. Kinet., 46, No. 9, 512–542 (2014).
Z. Hong, K. Y. Lam, D. F. Davidson, and R. K. Hanson, A comparative study of the oxidation characteristics of cyclohexane, methylcyclohexane, and n-butylcyclohexane at high temperatures, Combust. Flame, 158, No. 8, 1456–1468 (2011).
E. J. Silke, W. J. Pitz, C. K. Westbrook, and M. Ribaucour, Detailed chemical kinetic modeling of cyclohexane oxidation, J. Phys. Chem. A, 111, No. 19, 3761–3775 (2007).
W. J. Pitz, C. V. Naik, T. N. Mhaolduin, C. K. Westbrook, H. J. Curran, J. P. Orme, and J. M. Simmie, Modeling and experimental investigation of methylcyclohexane ignition in a rapid compression machine, Proc. Combust. Inst., 31, No. 1, 267–275 (2007).
F. Zhang, Z. Wang, Z. Wang, L. Zhang, Y. Li, and F. Qi, Kinetics of decomposition and isomerization of methylcyclohexane: Starting point for studying monoalkylated cyclohexanes combustion, Energy Fuels, 27, No. 3, 1679–1687 (2013).
S. A. Skeen, B. Yang, A. W. Jasper, W. J. Pitz, and N. Hansen, Chemical structures of low-pressure premixed methylcyclohexane flames as benchmarks for the development of a predictive combustion chemistry model, Energy Fuels, 25, No. 12, 5611–5625 (2011).
Z. Wang, H. Bian, Y. Wang, L. Zhang, Y. Li, F. Zhang, and F. Qi, Investigation on primary decomposition of ethylcyclohexane at atmospheric pressure, Proc. Combust. Inst., 35, No. 1, 367–375 (2015).
W. Sun, A. Hamadi, S. Abid, N. Chaumeix, and A. Comandini, A comparative kinetic study of C 8–C 10 linear alkylbenzenes pyrolysis in a single-pulse shock tube, Combust. Flame, 221, No. 1, 136–149 (2020).
W. Yuan, Y. Li, P. Dagaut, J. Yang, and F. Qi, Investigation on the pyrolysis and oxidation of toluene over a wide range conditions. I. Flow reactor pyrolysis and jet stirred reactor oxidation, Combust. Flame, 162, No. 1, 3–21 (2015).
K. Zhang, Q. Xin, Z. Mu, Z. Niu, and Z. Wang, Numerical simulation of diesel combustion based on n-heptane and toluene, Propul. Power Res., 8, No. 2, 121–127 (2019) .
C. S. McEna lly, L. D. Pfefferle, B. Atakan, and K. Kohse-Höinghaus, Studies of aromatic hydrocarbon formation mechanisms in flames: Progress towards closing the fuel gap, Prog. Energy Combust. Sci., 32, No. 3, 247–294 (2006).
A. Matsugi and A. Miyoshi, Modeling of two- and three-ring aromatics formation in the pyrolysis of toluene, Proc. Combust. Inst., 34, No. 1, 269–277 (2013).
L. V. Gurvich, IVTANTERMO — An automated system of data on thermodynamic properties of substances, Vestn. Akad. Nauk SSSR, No. 3, 54–65 (1983).
A. Burcat and B. Ruscic, Third Millennium Ideal Gas and Condensed Phase Thermochemical Database for Combustion with Updates from Active Thermochemical Tables, Report No. ANL-05/20 and TAE 960, Technion-IIT, Aerospace Engineering, and Argonne National Laboratory, Chemistry Division, Department of Energy, Oak Ridge: U.S. (2005).
J. Troe, From quantum chemistry to dissociation kinetics: What we need to know, Mol. Phys., 112, No. 18, 2374–2383 (2014).
M. Frenklach, Z. Liu, R. I. Singh, G. R. Galimova, V. N. Azyazov, and A. M. Mebel, Detailed, sterically-resolved modeling of soot oxidation: Role of O atoms, interplay with particle nanostructure, and emergence of inner particle burning, Combust. Flame, 188, 284–306 (2018).
V. S. Krasnoukhov, D. P. Porfiriev, I. P. Zavershinskiy, V. N. Azyazov, and A. M. Mebel, Kinetics of the CH3 + C5H5 reaction: A theoretical study, J. Phys. Chem. A, 121, No. 48, 9191–9200 (2017).
L. Zhao, T. Yang, R. I. Kaizer, T. P. Troy, M. Ahmed, D. Belisario-Lara, J. M. Ribeiro, and A. M. Mebel, A Combined experimental and computational study on the unimolecular decomposition of JP-8 jet fuel surrogates I: n-Decane (n-C10H22), J. Phys. Chem. A, 121, No. 6, 1261–1280 (2017).
P. Liu, Z. Li, A. Bennett, H. Lin, S. M. Sarathy, and W. L. Roberts, The site effect on PAHs formation in HACA-based mass growth process, Combust. Flame, 199, No. 1, 54–68 (2019).
F. Esposito and I. Armenise, Reactive, inelastic, and dissociation processes in collisions of atomic nitrogen with molecular oxygen, J. Phys. Chem. A, 125, No. 18, 3953–3964 (2021).
R. W. Bates, D. M. Golden, R. K. Hanson, and C. T. Bowman, Experimental study and modeling of the reaction H + O2 + M → HO2 + M (M = Ar, N2, H2O) at elevated pressures and temperatures between 1050 and 1250 K, Phys. Chem. Chem. Phys., 3, No. 12, 2337–2342 (2001).
Z. Hong, D. F. Davidson, E. A. Barbour, and R. K. Hanson, A new shock tube study of the H + O2 → OH + O reaction rate using tunable diode laser absorption near 2.5 μm, Proc. Combust. Inst., 33, No. 1, 309–316 (2011).
K. Sun, S. Wang, R. Sur, X. Chao, J. B. Jeffries, and R. K. Hanson, Sensitive and rapid laser diagnostic for shock tube kinetics studies using cavity-enhanced absorption spectroscopy, Opt. Express., 22, No. 8, 9291–9300 (2014).
R. K. Hanson and D. F. Davidson, Recent advances in laser absorption and shock tube methods for studies of combustion chemistry, Prog. Energy Combust. Sci., 44, 103–114 (2014).
N. M. Vandewiele, K. M. Van Geem, M. F. Reyniers, and G. B. Marin, Genesys: Kinetic model construction using chemo-informatics, Chem. Eng. J., 207–208, 526–538 (2012).
F. Sultan, M. Shahzad, and M. Ali, Spectral quasi equilibrium manifold and intrinsic low dimensional manifold: A multi-step reaction mechanism, Int. Commun. Heat Mass Transf., 121, No. 105098 (2021).
G. N. Simm, A. C. Vaucher, and M. Reiher, Exploration of reaction pathways and chemical transformation networks, J. Phys. Chem. A, 123, No. 2, 385–389 (2019).
J. A. Miller, R. Sivaramakrishnan, Y. Tao, C. F. Goldsmith, M. P. Burke, A. W. Jasper, N. Hansen, N. J. Labbe, P. Glarborg, and J. Zádor, Combustion chemistry in the twenty-first century: Developing theory-informed chemical kinetics models, Prog. Energy Combust. Sci., 83, Article ID 100886 (2021).
R. P. Lindstedt and L. Q. Maurice, Detailed chemical-kinetic model for aviation fuels, J. Propul. Power, 16, No. 2, 187–195 (2000).
G. Bikas and N. Peters, Kinetic modeling of n-decane combustion and autoignition, Combust. Flame, 126, Nos. 1–2, 1456–1475 (2001).
A. Ristory, P. Dagaut, and M. Cathonnet, The oxidation of n-hexadecane: Experimental and detailed kinetic modeling, Combust. Flame, 125, No. 3, 1128–1137 (2001).
S. Tanaka, F. Ayala, and J. C. Keck, A reduced chemical kinetic model for HCCI combustion of primary reference fuels in a rapid compression machine, Combust. Flame, 133, No. 4, 467–481 (2003).
M. Mehl, W. J. Pitz, C. K. Westbrook, and H. J. Curran, Kinetic modeling of gasoline surrogate components and mixtures under engine conditions, Proc. Combust. Inst., 33, No. 1, 193–200 (2011).
H. R. Zhang, E. G. Eddings, and A. F. Sarofim, Criteria for selection of components for surrogates of natural gas and transportation fuels, Proc. Combust. Inst., 31, No. 1, 401–409 (2007).
F. Buda, B. Heyberger, R. Fournet, P. A. Glaude, V. Warth, and F. Battin-Leclerc, Modeling of the gas-phase oxidation of cyclohexane, Energy Fuels, 20, No. 4, 1450–1459 (2006).
J. F. Griffiths, R. Piazzesi, E. M. Sazhina, S. S. Sazhin, P. A. Glaude, and M. R. Heikal, CFD modelling of cyclohexane auto-ignition in an RCM, Fuel, 96, No. 1, 192–203 (2012).
Z. Wang, L. Ye, W. Yuan, L. Zhang, Y. Wang, Z. Cheng, F. Zhang, and F. Qi, Experimental and kinetic modeling study on methylcyclohexane pyrolysis and combustion, Combust. Flame, 161, No. 1, 84–100 (2014).
W. Yuan, Y. Li, P. Dagaut, J. Yang, and F. Qi, Investigation on the pyrolysis and oxidation of toluene over a wide range conditions. II. A comprehensive kinetic modeling study, Combust. Flame, 162, No. 1, 22–40 (2015).
W. Yuan, Y. Li, G. Pengloan, C. Togbe, P. Dagaut, and F. Qi, A comprehensive experimental and kinetic modeling study of ethylbenzene combustion, Combust. Flame, 166, No. 1, 255–265 (2016).
S. Xi, J. Xue, F. Wang, and X. Li, Reduction of large-size combustion mechanisms of n-decane and n-dodecane with an improved sensitivity analysis method, Combust. Flame, 222, No. 1, 326–335 (2020).
Y. Chang, M. Jia, Y. Liu, Y. Li, and M. Xie, Development of a new skeletal mechanism for n-decane oxidation under engine-relevant conditions based on a decoupling methodology, Combust. Flame, 160, No. 8, 1315–1332 (2013).
N. A. Slavinskaya, Skeletal mechanism for kerosene combustion with PAH production, AIAA Paper, No. 2008-992 (2008).
W. Zeng, S. Liang, H.-X. Li, and H.-A. Ma, Chemical kinetic simulation of kerosene combustion in an individual flame tube, J. Adv. Res., 5, 357–366 (2014).
K. L. Tay, W. Yang, B. Mohan, H. A. D. Zhou, and W. Yu, Development of a robust and compact kerosene–diesel reaction mechanism for diesel engines, Energy Convers. Manage., 108, No. 1, 446–458 (2016).
N. Zettervall, C. Fureby, and E. J. K. Nilsson, A small skeletal kinetic mechanism for kerosene combustion, Energy Fuels, 30, No. 11, 9801–9813 (2016).
W. Yao, Y. Yuan, X. Li, J. Wang, K. Wu, and X. Fan, Comparative study of elliptic and round scramjet combustors fueled by RP-3, J. Propul. Power, 34, No. 3, 772–786 (2018).
Y. Yan, Y. Liu, W. Fang, Y. Liu, and J. Li, A simplified chemical reaction mechanism for two-component RP-3 kerosene surrogate fuel and its verification, Fuel, 227, No. 1, 127–134 (2018).
M. Jia and M. Xie, A chemical kinetics model of iso-octane oxidation for HCCI engines, Fuel, 85, Nos. 17–18, 2593–2604 (2006).
F. Maroteaux, Development of a two-part n-heptane oxidation mechanism for two stage combustion process in internal combustion engines, Combust. Flame, 186, No. 1, 1–16 (2017).
A. Vandersickel, Y. M. Wright, and K. Boulouchos, Global reaction mechanism for the auto-ignition of full boiling range gasoline and kerosene fuels, Combust. Theor. Model., 17, No. 6, 1020–1052 (2013).
B. Franzelli, E. Riber, M. Sanjose, and T. Poinsot, A two-step chemical scheme for kerosene–air premixed flames, Combust. Flame, 157, No. 7, 1364–1373 (2010).
T.-S. Wang, Thermophysics characterization of kerosene combustion, J. Thermophys. Heat Transf., 15, No. 2, 140–147 (2001).
J.-Y. Choi, A quasi-global mechanism of kerosene combustion for propulsion applications, AIAA Paper, No. 2011-5853 (2011).
A. V. Fedorov, D. A. Tropin, O. G. Penyazkov, V. V. Leshchevich, and S. Yu. Shimchenko, Theoretical and experimental study of chemical transformations of a methane–hydrogen–coal particles mixture in a rapid-compression machine, J. Eng. Phy s. Thermophys., 90, No. 4, 781–788 (2017).
V. A. Alekseev , J. V. Solovio va-Sokolova, S. S. Matveev, I. V. Chechet, S. G. Matveev, and A. A. Konnov, Laminar burning velocities of n-decane and binary kerosene surrogate mixture, Fuel, 187, No. 1, 429–434 (2017).
U. Pfahl, K. Fieweger, and G. Adomeit, Self-ignition of diesel–relevant hydrocarbon–air mixtures under engine conditions, Symp. (Int.) Combust., 26, No. 1, 781–789 (1996).
E. Olchanski and A. Burcat, Dacane oxidation in a shock tube, Int. J. Chem. Kinet., 38, No. 12, 703–713 (2006).
S. S. Vasu, D. F. Davidson, and R. K. Hanson, Jet fuel ignition delay times: Shock tube experiments over wide conditions and surrogate model predictions, Combust. Flame, 152, Nos. 1–2, 125–143 (2008).
H.-P. S. Shen, J. Steinberg, J. Vanderover, and M. A. Oehlschlaeger, A shock tube study of the ignition of n-heptane, n-decane, n-dodecane, and n-tetradecane at elevated pressures, Energy Fuels, 23, No. 5, 2482–2489 (2009).
H. Wang and M. A. Oehlschlaeger, Autoignition studies of conventional and Fischer–Tropsch jet fuels, Fuel, 98, No. 1, 249–258 (2012).
D. R. Haylett, D. F. Davidson, and R. K. Hanson, Ignition delay times of low-vapor-pressure fuels measured using an aerosol shock tube, Combust. Flame, 159, No. 2, 552–561 (2012).
V. P. Zhukov, V. A. Sechenov, and A. Yu. Starikovskiy, Autoignition of kerosene (Jet-A)/air mixtures behind reflected shock waves, Fuel, 126, 169–176 (2014).
H. S. Han, C. J. Kim, C. H. Cho, C. H. Sohn, and J. Han, Ignition delay time and sooting propensity of a kerosene aviation jet fuel and its derivative blended with a bio-jet fuel, Fuel, 232, 724–728 (2018).
B. Sirjean, F. Buda, H. Hakka, P. A. Glaude, R. Fournet, V. Warth, F. Battin-Leclerc, and M. Ruiz-Lopez, The autoignition of cyclopentane and cyclohexane in a shock tube, Proc. Combust. Inst., 31, No. 1, 277–284 (2007).
S. S. Vasu, D. F. Davidson, Z. Hong, and R. K. Hanson, Shock tube study of methyl-cyclohexane ignition over a wide range of pressure and temp erature, Energy Fuels, 23, No. 1, 175–185 (2009).
D. F. Davidson, B. M. Gauthier, and R. K. Hanson, Shock tube ignition measurements of iso-octane/air and toluene/air at high pressures, Proc. Combust. Inst., 30, No. 1, 1175–1182 (2005).
H.-P. S. Shen and M. A. Oehlschlaeger, The autoignition of C8H10 aromatics at moderate temperatures and elevated pressures, Combust. F lame, 156, No . 5, 1053–106 2 (2009).
D. Darcy, C. J. Tobin, K. Yasunaga, J. M. Simmie, J. Würmel, W. K. Metcalfe, N. Niass, S. S. Ahmed, C. K. Westbrook, and H. J. Curran, A high pressure shock tube study of n-propylbenzene oxidation and its comparison with n-butylbenzene, Combust. Flame, 159, No. 7, 2219–2232 (2012).
E. Ranzi, A. Frassoldati, R. Grana, A. Cuoci, T. Faravelli, A. Kelley., and C. Law, Hierarchical and comparative kinetic modeling of laminar flame speeds of hydrocarbon and oxygenated fuels, Prog. Energy Combust. Sci., 38, No. 4, 468–501 (2012).
Z. Zhao, J. Li, A. Kazakov, F. L. Dryer, and S. P. Zeppieri, Burning velocities and a high-temperature skeletal kinetic model for n-decane, Combust. Sci. Technol., 177, No. 1, 89–106 (2004).
K. Kumar and C.-J. Sung, Laminar flame speeds and extinction limits of preheated n-decane/O2/N2 and n-dodecane/O2/N2 mixtures, Combust. Flame, 151, Nos. 1–2, 209–224 (2007).
A. Moghaddas, K. Eisazadeh-Far, and H. Metghalchi, Laminar burning speed measurement of premixed n-decane/air mixtures using spherically expanding flames at high temperatures and pressures, Combust. Flame, 159, No. 4, 1437–1443 (2012).
F. Luo, W. Song, W. Chen, and Y. Long, Investigation of kerosene supersonic combustion performance with hydrogen addition and fuel additive at low Mach inflow conditions, Fuel, 285, Article ID 119139, 1–13 (2021).
G. Borghesi, A. Krisman, T. Lu, and J. H. Chen, Direct numerical simulation of a temporally evolving air/n-dodecane jet at low-temperature diesel-relevant conditions, Combust. Flame, 195, No. 1, 183–202 (2018).
Yu. V. Tunik, G. Ya. Gerasimov, V. Yu. Levashov, and N. A. Slavinskaya, Numerical modeling of detonation combustion of a kerosene vapor in a divergent nozzle, Fiz. Goreniya Vzryva, 56, No. 3, 106–114 (2020).
Y. V. Tunik, Numerical solution of test problems using a modified Godunov scheme, Comp. Math. Math. Phys., 58, No. 10, 1573–1584 (2018).
Y. Yan, Y. Liu, D. Di, C. Dai, and J. Li, Simplified chemical reaction mechanism for surrogate fuel of aviation kerosene and its verification, Energy Fuels, 30, No. 12, 10847–10857 (2016).
G. Eckel, J. Grohmann, L. Cantu, N. Slavinskaya, T. Kathrotia, M. Rachner, P. Le Clercq, W. Meier, and M. Aigner, LES of a swirl-stabilized kerosene spray flame with a multi-component vaporization model and detailed chemistry, Combust. Flame, 207, No. 1, 134–152 (2019).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Inzhenerno-Fizicheskii Zhurnal, Vol. 97, No. 2, pp. 515–534, March–April, 2024.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gerasimov, G.Y., Levashov, V.Y. Kinetic Models of Combustion of Kerosene. J Eng Phys Thermophy 97, 506–524 (2024). https://doi.org/10.1007/s10891-024-02918-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10891-024-02918-x