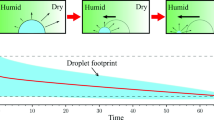
An advantage of spray technologies in polycondensation processes is the efficient removal of low-molecular-weight products and heat. These advantages are based on dimensional effects which may lead a substantial shift of chemical equilibrium in reversible reactions. In this work, within the framework of formal chemical kinetics, the authors have modeled the dependence of the polycondensation rate on the droplet size, the composition of the reactive mixture, and the gas medium. This dependence was reproduced experimentally using the polycondensation of lactic acid as an example. Based on the results of microscopic observation of the behavior of an ensemble of sessile droplets of aqueous and aqueous-alcoholic solutions of lactic acid, the authors have constructed kinetic curves of contraction of droplets of varying size. The contraction is a result of the set of interrelated external and intradiffusion and intrakinetic processes and phase transformations whose limiting stage is the polymerization rate. The described regularities are thermodynamic in nature and must be manifested in polymerization and polycondensation with the participation of volatile monomers and solvents.
Similar content being viewed by others
References
U. Fritsching (Ed.), Process-Spray: Functional Particles Produced In Spray Processes, Springer, Cham (2016).
A. Moridi, S. M. Hassani-Gangaraj, M. Guagliano, and M. Dao, Cold spray coating: Review of material systems and future perspectives, Surf. Eng., 30, 369–395 (2014); https://doi.org/10.1179/1743294414Y.0000000270.
J. Deng, X. Ding, W. Zhang, Y. Peng, J. Wang, X. Long, P. Li, and A. S. Chan, Magnetic and conducting Fe3O4 — Cross-linked polyaniline nanoparticles with core–shell structure, Polymer (Guild), 43, 2179–2184 (2002); https://doi.org/10.1016/S0032-3861(02)00046-0.
M. Shaban, J. Poostforooshan, and A. P. Weber, Surface-initiated polymerization on unmodified inorganic semiconductor nanoparticles: Via surfactant-free aerosol-based synthesis toward core-shell nanohybrids with a tunable shell thickness, J. Mater. Chem. A, 5, 18651–18663 (2017); https://doi.org/10.1039/c7ta04985d.
J.-H. Park, C. Oh, S.-I. Shin, S.-K. Moon, and S.-G. Oh, Preparation of hollow silica microspheres in W/O emulsions with polymers, J. Colloid Interface Sci., 266, 107–114 (2003); https://doi.org/10.1016/S0021-9797(03)00645-3.
V. Marturano, P. Cerruti, M. Giamberini, B. Tylkowski, and V. Ambrogi, Light-responsive polymer micro- and nanocapsules, Polymers, 9, No. 8 (2016); https://doi.org/10.3390/polym9010008.
J. Raula, H. Eerikäinen, and E. I. Kauppinen, Influence of the solvent composition on the aerosol synthesis of pharmaceutical polymer nanoparticles, Int. J. Pharm., 284, 13–21 (2004); https://doi.org/10.1016/j.ijpharm.2004.07.003.
C. Wu, A. Ying, and S. Ren, Fabrication of polymeric micelles with core–shell–corona structure for applications in controlled drug release, Colloid Polym. Sci., 291, 827–834 (2013); https://doi.org/10.1007/s00396-012-2794-8.
C. Cheng, F. N. Mutua, Y. Dong, B. Zhu, and Y. He, Bio-based poly(Pentamethylene oxamide) synthesized by spray/solid-state polycondensation, Polym. Bull., 75, 121–134 (2018); https://doi.org/10.1007/s00289-017-2023-1.
B. Li, H. Lopez-Beltran, C. Siu, K. H. Skorenko, H. Zhou, W. E. Bernier, M. S. Whittingham, and W. E. Jones, Vapor phase polymerized PEDOT/cellulose paper composite for flexible solid-state supercapacitor, ACS Appl. Energy Mater., 3, 1559–1568 (2020); https://doi.org/10.1021/acsaem.9b02044.
E. Akgün, A. Muntean, J. Hubbuch, M. Wörner, and M. Sangermano, Cationic aerosol photopolymerization, Macromol. Mater. Eng., 300, 136–139 (2015); https://doi.org/10.1002/mame.201400211.
E. N. Fedoseeva and V. B. Fedoseev, Possibilities and peculiarities of spray technologies in organic synthesis, Condens. Matter Interphases, 22, 397–405 (2020); https://doi.org/10.17308/kcmf.2020.22/3001.
V. B. Fedoseev and E. N. Fedoseeva, Size effects during phase transformations in stratifying systems, Russ. J. Phys. Chem. A, 88, 436–441 (2014); https://doi.org/10.1134/S0036024414020083.
A. V. Shishulin and V. B. Fedoseev, Features of the influence of the initial composition of organic stratified mixtures in microsized pores on the mutual solubility of components, Tech. Phys. Lett., 46, 938–941 (2020); https://doi.org/10.1134/S1063785020090291.
V. B. Fedoseev, Size effects in delamination of poly(methyl methacrylate)–acetone–hexane solution, Polym. Sci. Ser. A, 63, 445–450 (2021); https://doi.org/10.1134/S0965545X21050047.
O. G. Penyazkov, V. I. Saverchenko, and S. P. Fisenko, Low-temperature synthesis of nanoparticles in the process of evaporation of femtoliter droplets of a solution at a low pressure, J. Eng. Phys. Thermophys., 87, No. 4, 796–801 (2014); https://doi.org/10.1007/s10891-014-1074-5.
V. B. Fedoseev and E. N. Fedoseeva, Polycondensation in a spray of aqueous-alcoholic solution of lactic acid, Condens. Matter Interphases, 23, 101–108 (2022); https://doi.org/10.17308/kcmf.2022.24/9060.
A. G. Morozov, I. L. Fedyushkin, and D. Y. Aleinik, L-Lactide ring-opening polymerization on a magnesium catalyst based on acenaphthene-1,2-diimine ligand: Development of biocompatible materials for osteoplasty, Russ. J. Appl. Chem., 89, 2095–2101 (2016); https://doi.org/10.1134/S1070427216120247.
Yu. E. Pokharukova, V. T. Novikov, and V. N. Glotova, Polycondensation of lactic acid to an oligomer in a solution, Vestn. Kuzbassk. Gos. Tekh. Univ., 118, No. 1, 134–138 (2017).
Ren Jie (Ed.), Biodegradable Poly (Lactic Acid): Synthesis, Modification, Processing and Applications, Springer Science & Business Media (2011).
Ya. B. Zel′dovich, On the Theory of the Reaction on a Porous or Powder Material, Chemical Physics and Hydrodynamics. Selected Works [in Russian], Nauka, Moscow (1984), pp. 65–70.
D. A. Frank-Kamenetskii, The Principles of Macrokinetics. Diffusion and Heat Transfer in Chemical Kinetics, 4th edn. [in Russian], Izd. Dom “Intellekt,” Dolgoprudnyi (2008).
V. N. Emel'yanenko, S. P. Verevkin, C. Schick, E. N. Stepurko, G. N. Roganov, and M. K. Georgieva, The thermodynamic properties of S-lactic acid, Russ. J. Phys. Chem. A, 84, 1491–1497 (2010).
V. B. Fedoseev and E. N. Fedoseeva, Formation of bi- and polymodal distributions and the non-Ostwald behavior of disperse systems, J. Eng. Phys. Thermophys., 92, No. 5, 1191–1200 (2019); https://doi.org/10.1007/s10891-019-02033-2.
Y. M. Harshe, G. Storti, M. Morbidelli, S. Gelosa, and D. Moscatelli, Polycondensation kinetics of lactic acid, Macromol. React. Eng., 1, 611–621 (2007); https://doi.org/10.1002/mren.200700019.
M. B. Gawande, A. Goswami, T. Asefa., H. Guo, A. V. Biradar, D.-L. Peng, R. Zboril, and R. S. Varma, Core–shell nanoparticles: Synthesis and applications in catalysis and electrocatalysis, Chem. Soc. Rev., 44, 7540–7590 (2015); https://doi.org/10.1039/C5CS00343A.
K. W. Kim and S. I. Woo, Synthesis of high-molecular-weight poly(L-lactic acid) by direct polycondensation, Macromol. Chem. Phys., 203, No. 15, 2245–2250 (2002); https://doi.org/10.1002/1521-3935(200211)203:15<2245::AIDMACP2245>3.0.CO;2-3.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Inzhenerno-Fizicheskii Zhurnal, Vol. 96, No. 5, pp. 1204–1212, September–October, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fedoseev, V.B., Fedoseeva, E.N. Kinetics of Polycondensation in a Spray of Aqueous and Aqueous-Alcoholic Solutions of Lactic Acid. J Eng Phys Thermophy 96, 1196–1204 (2023). https://doi.org/10.1007/s10891-023-02785-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10891-023-02785-y