Abstract
Terpenes are a major class of secondary metabolites present in all plants, and long hypothesized to have diversified in response to specific plant-herbivore interactions. Herbivory is a major biotic interaction that plays out across broad temporal and spatial scales that vary dramatically in temperature regimes, both due to climatic variation across geographic locations as well as the effect of seasonality. In addition, there is an emerging understanding that global climate change will continue to alter the temperature regimes of nearly every habitat on Earth over the coming centuries. Regardless of source, variation in temperature may influence herbivory, in particular via changes in the efficacy and impacts of plant defensive chemistry. This study aims to characterize temperature-driven variation in toxicological effects across several structural classes of terpenes in the model herbivore Vanessa cardui, the painted lady butterfly. We observed a general increase in monoterpene toxicity to larvae, pupa, and adults at higher temperatures, as well as an increase in development time as terpene concentration increased. Results obtained from this study yield insights into possible drivers of seasonal variation in plant terpene production as well as inform effects of rising global temperatures on plant-insect interactions. In the context of other known effects of climate change on plant-herbivore interactions like carbon fertilization and compensatory feeding, temperature-driven changes in plant chemical defense efficacy may further complicate the prediction of climate change impacts on the fundamental ecological process of herbivory.
Similar content being viewed by others
Introduction
To deter herbivory, plants possess sophisticated and diverse arsenals of chemical defenses composed of many individual secondary metabolites. Plant secondary metabolites are ubiquitous in the plant kingdom and have a wide range of ecological roles such as mediating symbiotic interactions and plant-plant signaling, direct defense against herbivores and pathogens, and providing protection against abiotic stressors such as frost and drought (Dixon and Paiva 1995; Gershenzon and Dudareva 2012). Chemical defenses can be produced constitutively or induced upon attack to directly and indirectly thwart herbivore attacks by exerting toxic effects upon contact or ingestion as well as by attracting natural predators of herbivores (Howe and Jander 2008).
One major class of secondary metabolites are the terpenes, which have exceptional abundance and diversity in flowering plants. The majority of the diversity in terpenoids is lineage specific - of the 25,000 terpenes reported, only about 100 are present across all plant species (Tissier et al. 2014). An explanation for the abundance of lineage specific compounds is the evolutionary arms race proposed by Ehrlich and Raven (1964) in which existing molecules are modified to create novel compounds unexperienced by herbivores. The most well-established function for terpenes is defense against biological enemies through direct and indirect defense, but assigning specific roles is difficult to establish because multiple terpenes are often expressed at once in volatile bouquets (Pichersky and Raguso 2018). For those with direct roles in chemical defense against herbivores, terpenes have been shown to decrease the likelihood of survival to reproduction and slow the rate of larval growth (Kumbasli and Bauce 2013). In lepidopteran larvae, terpenes block the stimulatory effects of glucose and inositol on chemosensory receptor cells located on the mouthparts (Gershenzon and Dudareva 2012). Several monoterpenoids, including linalool and limonene, may be neurotoxic or act as insect growth regulators, disrupting the normal process of morphogenesis (Balandrin and Klocke 1988; Coats et al. 1991). Leaf allelochemical composition, including terpene expression, is heavily influenced by both biotic and abiotic conditions (Kleiner 1989; Hunter 1992), and recent studies suggest that seasonality may regulate the production of a variety of defense compounds (Gols et al. 2018; Blanchard and Bowers 2020).
Although the extent to which seasonal changes such as daylength and temperature influence plant defenses remains elusive, their impacts on insects are extensively studied. Seasonal fluctuations in daylength, temperature, and host plant availability influence the metabolic activities of many short-lived herbivores, including insects. Much of insect life history revolves around favorable and unfavorable conditions for growth and reproduction, which many species rely on seasonal cues to detect. Developmental tactics such as diapause or dispersal via migration are strategies to avoid energy expenditures during unfavorable periods. Diapause permits insect survival under adverse climatic conditions and synchronizes the life cycles of individual insects within a population as well as with the phenology of their food supply (Chippendale 1982), and migration can permit insect species to continue to avoid adverse climatic conditions or a lack of food availability altogether (Hahn and Denlinger 2011, Chapman et al. 2015, Hu et al. 2021). Insects are cued to initiate diapause or migration when daylength, moisture, temperature, and foliage quality decline at the end of growing seasons. For example, the end of the monarch breeding season and beginning of their migration to southern overwintering grounds is signaled by the dormancy of their larval host plant, milkweed (Goehring and Oberhauser 2002). In many ecosystems, it is typical for plant phenology to temporally restrict the growth and development of specific phytophagous insects (Feeny 1970; Wint 1983).
The extensive history of coevolution between plants and insects has created seasonally-regulated synchronously-timed life events within communities, such as insect emergence being aligned with host leaf-out phenology, but global climate change is complicating these sensitive dynamics by altering the geographical ranges, population dynamics, and phenologies of many organisms (Bale et al. 2002). Desynchrony between emergence or hatching and host plant phenology can have disastrous and long-lasting impacts on both plant and herbivore populations (Yukawa and Akimoto 2006; Teder 2020). For herbivorous insects, emergence before sufficient host plant availability can result in intensive competition and mass mortality events (Forrest 2016; Fuentealba et al. 2017). Likewise, an increase in ambient temperature can have dramatic effects on developmental rates, reproductive potential, overwintering survival, and the number of generations occurring within a season (Ayres and Lombardero 2000). Global warming has created prolonged reproductive windows, which in turn can bolster insect populations (Stoeckli et al. 2012).
Larger herbivore populations present for a longer portion of the year paired with declining leaf nutrient quality will potentially increase the length and degree of herbivory pressure on plant populations (Marini et al. 2017). Assessment of herbarium specimens over the past century suggests that increasing temperatures have increased rates of herbivory in temperate plants, in parallel with increases in the predicted occurrence of many dozens of species of lepidopteran herbivores (Meineke et al. 2019). Multiple generations of herbivores can inflict unprecedented levels of damage over the course of a single season, particularly on long-lived plants such as pines (Porter et al. 1991). As global temperatures rise, instances of altered voltinism have already been observed in herbivorous insects. In one such study of 263 multivoltine central European lepidopteran species spanning the last 40 years, over half of species displayed an increased frequency of intra-year second and subsequent generations, and a quarter of species displayed constant increases in the number of generations per year (Altermatt 2010). Increased herbivore voltinism not only poses a substantial threat to the biodiversity of plant communities but also has broad implications for agricultural losses from pests (Hamann et al. 2021). In the case of the rice leaffolder (Cnaphalocrosis medinalis), warmer temperatures have been associated with increased outbreaks, which reduces rice yield potential and increases management costs (Ali et al. 2019). Destruction potential is further heightened as plant nutritional quality declines. Nitrogen, the primary nutritional element limiting insect growth, is known to be present at subaverage concentrations in foliage subject to elevated CO2 and temperature – which in turn stimulates insects to consume more foliage to compensate for reduced nutrient availability (Zvereva and Kozlov 2006). The evidence to date suggests that in the future recurring herbivore outbreaks are likely to become more frequent, and baseline herbivore pressure more destructive, due to temperature-associated developmental acceleration, increases in voltinism, and feeding stimulation.
As rising global temperatures increase the rate of development of insect herbivores, plant defensive secondary metabolites could directly counter this effect by inducing insect developmental delays. Likewise, feeding stimulation may be countered by increased rates of insect mortality upon ingestion of relatively larger doses of chemical defenses per unit of nutrients obtained. However, these counteracting effects may be undermined if the efficacy of chemical defenses against insect herbivores is also altered by temperature, for instance due to temperature effects on insect metabolism. As the majority of primary productivity enters food webs in terrestrial ecosystems through herbivory, understanding how the efficacy of plant chemical defenses will change with ambient temperature is key to making informed predictions about the response of plant-insect interactions to climate change.
To test the hypothesis that the efficacy of chemical defense is altered by temperature, we assess the temperature-dependent impacts of four common terpene defenses in the model insect herbivore Vanessa cardui (Lepidoptera: Nymphalidae) – known as the thistle caterpillar or painted lady butterfly. This is one of the most widely distributed butterflies in the world, absent only from South America and Antarctica. The duration of the life cycle ranges from 21 days to multiple months, driven by variation across its near-cosmopolitan distribution, as well as its migratory behavior, and breadth of diet. Vanessa cardui is highly polyphagous, feeding on host species spanning at least ten different plant orders (Celorio-Mancera 2016), and a serious agricultural pest of crops as diverse as corn, alfalfa, sunflowers, beans, and soy (Williams 1970). This species has also historically exhibited large fluctuations in population size over time and mass migrations of millions of individuals (Kelly and Debinski 1999; Hu et al. 2021), making the likelihood of rapid increases in insect abundance and resulting herbivory pressure likely.
Overall, this study asks two questions: (1) how does temperature alter the toxicity of terpene defenses to Vanessa cardui in terms of mortality and insect mass, and (2) how do temperature and dietary terpene concentration affect the rate of Vanessa cardui development?
Methods
Experimental design
In order to assess how temperature alters the efficacy of terpene defenses, controlled experiments were performed manipulating both temperature and dietary terpene concentration and recording mortality, adult mass, and developmental responses of V. cardui. In addition to its relevant ecology, this species is easy to rear in laboratory settings and thrives on general lepidopteran diets (Ahmad et al. 1989). Individual insects were reared from eggs (Carolina Biological Supply Company, Burlington, NC), in individual 1.25-ounce plastic cups (Frontier Agricultural Sciences, Newark, DE) on optimized artificial V. cardui diet (Frontier Agricultural Sciences, Newark, DE). We chose commercially sourced V. cardui to avoid issues such as presence of disease and temporally-limited abundance that are often experienced when using field-sourced specimens (Kelly and Debinski 1999). Rearing insects, including V. cardui, on artificial diet is common practice in a broad range of applications such as transcriptomics, pest management, and biocontrol studies (Connahs et al. 2016; Sørensen et al. 2012; Li et al. 2014). Artificial diet was adulterated with one of four selected terpenes at ten concentrations including a control with no terpenes present (Datasets S1-S3), as informed by preliminary pilot trials for each compound conducted at room temperature. To adequately represent a cross-section of terpene diversity, four botanically common terpenoids belonging to different structural subclasses were tested: D-limonene (cyclic monoterpene, CAS# 5989-27-5, concentrations from 0-12%), 1,8-cineole (cyclic monoterpene ether, CAS# 470-82-6, concentrations from 0-9%), linalool (acyclic monoterpene alcohol, CAS# 78-70-6, concentrations from 0-2.5%), and beta-caryophyllene (bicyclic sesquiterpene, CAS# 87-44-5, concentrations from 0-6%). Concentrations differed among the four terpenes to adequately span sublethal to lethal concentrations, and the concentrations used for each terpene were not kept identical between trials, as multiple uninformative high doses with full mortality in early trials were replaced in later trials with additional intermediate doses for higher precision of estimated toxicity (see Dataset S1-S3). The ranges of concentrations used in this study span reported plant tissue concentrations for terpenoids generally (Blanch et al. 2009; Kopaczyk et al. 2020), and for these terpenoids in particular (King et al. 2006; Mewalal et al. 2017; Song et al. 2019; Saunier et al. 2022).
Terpenoids were purchased from Fisher Scientific (Waltham, MA) as liquids of high percent purity (96-99%) and homogenized with diet media in a blender. Diet was prepared 4-7 days prior to insect placement, with 0.5 liters prepared per treatment dose and approximately 17 grams distributed into each replicate rearing cup (slightly over half of the cup volume). Prepared cups were then stored at 4°C with sealed lids to prevent terpene loss due to volatilization. Insects were reared in climate-controlled incubators (136LL, Percival, Inc) for the duration of their life cycle at a L12:D12 photoperiod and at one of three different temperatures, 24°C, 27°C, or 30°C, to represent the lower threshold, median temperature, and upper threshold at which V. cardui is able to successfully complete its full life cycle (Pakyari et al. 2019; Poston et al. 1977). A single trial run consisted of a total of 400 individuals - four terpenes at ten concentrations with ten replicate insects at each concentration – reared from egg to adult at a single temperature. Two replicate trial runs were performed at each temperature, for a total of six trial runs overall. The focal response variables were rate of hatching failure, larval mortality, pupa mortality, adult mortality, development time from hatching to pupation and emergence (eclosure), and adult dry biomass.
Specimen rearing and scoring procedures
Specimens were reared from eggs, following typical insect ecotoxicology procedures. For each diet containing cup, three eggs were placed onto wax paper to separate them from direct contact with the surface of the diet, otherwise diet media would interfere with the outer egg membrane and hatching could not occur. A two-ply tissue paper was placed between the cup opening and lid as an attachment point for the chrysalis cremaster at pupation. Approximately eight small airholes were made on the lid of each cup to permit gas exchange. These cups were then placed into their respective growth chambers, using a randomized spatial design to assign individual larvae to shelves. Beginning the second week of the trial, 40 μl of water was added to the surface of diets each week to maintain optimum diet moisture. Once hatching occurred (typically one to two days) additional larvae were removed along with the residing wax paper so only one out of the three original caterpillars remained in each cup. For diet treatments that fully inhibited hatching within a cup, additional larvae that had been reared on control diet in parallel to experimental specimens were used as replacements for the absent larvae, to assess terpene toxicity in the following development stages. Small paintbrushes were used to transfer these replacement larvae onto the terpene treated diets that inhibited hatching, occurring approximately three days after hatching, depending on temperature, when they were robust enough to tolerate manipulation. Once chrysalis formation occurred for a replicate insect, the tissue paper upon which the cremaster was secured was pinned to the top of a standard 28 L netted flight box to permit completion of the life cycle to adult emergence. Similar to larval cups, flight boxes were placed into their respective incubators and assigned to shelves using a randomized spatial design. Since space in growth chambers was limited and each pupa could not be allotted their own individual flight box, specimens were grouped by the specific treatment diet on which they were reared (by concentration within each compound).
Mortality and stage of development were recorded at least every other day. The date of hatching commenced development tracking and was considered day 0 when counting days till the next development stage was reached. The date of chrysalis formation marked the transition from larval to pupal phase, and date of emergence marked the transition from pupal phase into adulthood. Individual specimens were reared alone in a single container from egg to pupa so scoring data could be assigned to those individuals with certainty, whereas days to adulthood could only be estimated per specimen because pupa were transferred to flight chambers with others from their treatment. In cases where only one adult emerged from its chrysalis during a scoring interval (one to two days), days to adulthood could be known with certainty. In cases where multiple adults emerged during a scoring interval, days to adulthood could be estimated to within the scoring interval (one to two days). In cases where a pupa did not form any or only a weak cremaster, they were placed on the floor of the flight chamber and recorded as an improperly formed chrysalis. The development of these pupa could be easily tracked into adulthood because if they emerged, they were typically deformed and stuck in place due to improper wing drying. Since these adults were immobile, the date of their emergence could be confidently assigned to the corresponding deformed pupa in its place. Butterflies were subsequently placed into glassine envelopes with wings folded behind the ventral edge of the body and on either side of the abdomen, were then euthanized by freezing and stored in a desiccation chamber. Once all trials were completed and adult specimens had been desiccated to a stable dry mass, they were weighed for adult biomass.
Larval death was determined if larvae were unresponsive to physical stimulus such as light prodding with a clean paintbrush and unable to maintain surface attachment. If a specimen began chrysalis formation but was unable to complete chrysalis development to the extent emergence was biologically impossible, the specimen was determined to have died in the pupal stage. In this scenario, date of chrysalis formation and pupal death would be the same. If the specimen did form a functional chrysalis but did not emerge more than two weeks after the last observed emergence for its treatment group, it was also considered a pupal death. Adults with severely deformed or folded wings and that were unable to fly were considered adult deaths and excluded from adult biomass analysis. The mass of deformed adults tended to be much higher than normal adults because they retain meconium that is normally expelled from healthy adults during eclosure.
Statistical analysis
Linear mixed models with correlated random slopes and intercepts were run for each continuous trait (time to pupation, time to emergence, and adult dry mass). Generalized linear mixed models with random slopes and intercepts were run for binary traits (hatching failure, and cumulative mortality for larva, pupa, and adults) using a binomial distribution with a logit link. For generalized linear mixed effects models, correlations between random slopes and intercepts were unidentifiable, so these terms were fit as uncorrelated. All mixed effects models were run in the R package lme4 (Bates et al. 2014). For all traits, temperature (categorical), terpene concentration (continuous), and their interaction were all treated as fixed effects, whereas the temporally replicated trial run was treated as a random effect. With trial run as the grouping variable, concentration was modeled as a random slope to account for possible heterogeneity across runs. Fixed effects were resampled 10,000 times using the mvrnorm function in the MASS package (Venables and Ripley 2002) to compute 95% bootstrap confidence intervals for LC50s and regression coefficients. For linear mixed models, we visually assessed assumptions using the performance package (Lüdecke et al. 2021) to check normality of residuals and random effects, linearity, homogeneity of variance, and leverage. For generalized linear mixed models, we assessed model adequacy by assessing posterior predictive performance. For all models presented we did not observe any notable violations of assumptions. To generate figures, regression lines were plotted using the fixed effects estimates along a grid of predictor values, with the bounds of 95% confidence bands generated by extracting the fixed effects and their covariances for resampling. The median lethal concentration (LC50), represented as percent weight of terpene per total weight of diet (w/w %), was compared at all life stages (Table 1) and used as a metric of relative toxicity where a high LC50 represent low toxicity and a low LC50 represents high toxicity.
Results
Terpene toxicity
All terpenes tested caused an increase in hatching failure at higher concentrations except for beta-caryophyllene (Supplementary Fig. S1) which displayed irregular patterns of hatching inhibition at variable temperatures and concentrations. Beta-caryophyllene overall had the least influence on hatching, with inhibition rarely exceeding 50% at any concentration. Cineole displayed a significantly higher LC50 (i.e., non-overlapping 95% confidence intervals) at 30°C compared to the LC50 observed at 24°C or 27°C, but no difference was observed between 24°C and 27°C (Table 1; Supplementary Fig. S1), suggesting the efficacy of cineole to inhibit hatching decreases at higher temperatures and is greatest at low temperatures. Similarly, limonene and linalool both inhibited hatching most at the lowest temperature (24°C), with significantly lower LC50s than at higher temperature (Table 1, Supplementary Fig. S1). These results suggest that terpenes with roles in hatching inhibition are more powerful at lower temperatures.
The toxicity of the four terpenes in the larval stage were not statistically significantly different by temperature, but the estimated LC50 of both cineole and linalool showed a consistent decrease with increasing temperatures (Table 1, Supplementary Fig. S2). Limonene also had the highest LC50 at the lowest temperature but did not display the same consistent decrease with temperature as linalool and cineole. The sesquiterpene beta-caryophyllene demonstrates no significant differences in LC50 with temperature, nor any substantial trend. Opposite of the patterns observed for hatching failure, the three monoterpenes seem to be more toxic towards larvae at higher temperatures, although not significantly different.
Greater monoterpene toxicity at high temperatures continues in the pupal stage with mortality rates becoming more distinct. Linalool had a significantly lower LC50 at 30°C than both 24 and 27°C (Table 1, Supplementary Fig. S3). The LC50 for cineole were also significantly lower at 30°C than 24°C, with 27°C intermediate. The toxicity of beta-caryophyllene and limonene for the pupal stage did not vary significantly with temperature (Table 1, Supplementary Fig. S3), but the overall trend observed in larva for monoterpenes to have higher toxicity at higher temperatures continued in the pupa stage.
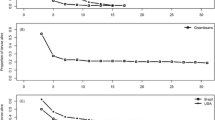
In general, adult stage mortality followed the same pattern as larval and pupal mortality, though 95% confidence intervals for LC50s of adult mortality typically overlapped across temperatures for limonene, cineole, and beta-caryophyllene (Table 1, Fig. 1). For linalool, the LC50s for 24°C and 30°C were significantly different, whereas the LC50 for 27°C was intermediate and not significantly different from either 24°C or 30°C (Table 1). The toxicity of linalool and cineole displayed the same trend as for larval and pupal mortality, consistently increasing from 24°C to 27°C to 30°C. However, the other two terpenes differed, with limonene having the lowest adult LC50 and greatest toxicity at 27°C (significantly more toxic at 27°C than 24°C), with 30°C intermediate (Table 1).
Concentration-response curves of adult mortality at 24°C, 27°C, and 30°C with V. cardui subject to varying concentrations of A) limonene, B) linalool, C) cineole, and D) beta-caryophyllene as prepared at the start of each trial. Shaded regions correspond to 95% confidence intervals around each curve. Individual points represent the proportion of adult mortality observed across replicate insects within each concentration for each trial. See Data S1 for full data
Developmental timing and adult body mass
The number of days to pupation and emergence was plotted against the dietary concentration of each terpene. The individual effect of temperature without the influence of terpenes can be evaluated by comparing y-intercepts (Table 2). Within a given temperature, 95% confidence intervals for y-intercepts for a given developmental event (pupation, emergence) overlapped in all but a single case (cineole, time to pupation at 27°C). This provides context that estimates of development time in non-treated controls were largely consistent across the entire experiment. Comparing y-intercepts across temperatures indicates that higher temperatures speed development, reducing the number of days to both pupation and emergence (Table 2). In all but one case, days to both pupation and emergence were significantly lower at 27°C than 24°C, with a similar magnitude of around 4-5 days (Table 2). Days to pupation and emergence were significantly lower at 30°C than 27°C in half of cases, with a typical magnitude of around 1-4 days regardless of statistical significance (Table 2). In all cases, development time at 30°C was significantly faster than 24°C, with time to development points (e.g. chrysalis formation and adult eclosure) being reached approximately six to eight days faster (Table 2). The magnitude of difference was roughly the same for both time to pupation and time to emergence, indicating that the increased pace of development occurred primarily in the larval stage while specimens were feeding.
Slopes of the relationship between dietary terpene concentration and days to pupation or emergence were compared to detect interactive effects between concentration and temperature. A positive slope that differs from zero indicates that increasing terpene concentration has a delaying effect on development. Further, a significant difference (nonoverlapping confidence intervals) between the slopes observed at two temperatures would indicate that this delaying effect changes with temperature. Time to pupation was observed to have a nonzero slope at all temperatures for beta-caryophyllene and limonene, and at the developmental thermal optimum of 27°C for cineole and linalool (Table 2). The magnitude of these delaying effects varies from roughly 1-9 days per unit terpene concentration (w/w %). Comparison of slopes for time to pupation across temperatures indicates that the magnitude of the delaying effect does not significantly change with temperature for beta-caryophyllene, cineole, or linalool, though is substantially stronger for limonene at the thermal optimum of 27°C than at other temperatures (Table 2). Estimates of slopes for time to emergence were observed to have wide confidence intervals due to smaller sample size (only a subset of individual reach adulthood due to mortality), yet significantly nonzero slopes were observed for cineole, limonene, and linalool at the thermal optimum of 27°C, and for beta-caryophyllene at 24°C (Table 2). Comparisons of slopes for time to emergence find no significant differences with temperature, other than a stronger delaying effect for beta-caryophyllene at 24°C than at higher temperatures (Table 2, Fig. 2).
Concentration versus days to pupation at 24°C, 27°C, and 30°C with V. cardui subject to varying concentrations of A) limonene, B) linalool, C) cineole, and D) beta-caryophyllene as prepared at the start of each trial. Shaded regions correspond to 95% confidence intervals around each curve. Individual points represent the time to pupation observed for each insect that reached pupation within each trial. See Data S2 for full data
Generally, adult body mass somewhat declined with increasing dietary terpene concentrations, but effects of temperature and terpene concentration were nonsignificant other than for limonene where the slope of the effect of terpene concentration on adult body mass was significant and negative for 24°C and 30°C (Supplementary Fig. S5). In effect, if V. cardui was able to successfully emerge from a chrysalis without visible deformation, average adult size varied within around a two-fold range.
Limitations to study design
The design choice to replace eggs that failed to hatch with newly hatched young larvae was made in order to enable assessment of post-hatching mortality and developmental timing across the life cycle. Our intent was to emulate oviposition onto a leaf and subsequent insect development on a host plant, and replacement upon hatching failure was conceptually considered to be analogous to a larvae crawling onto a focal leaf from adjacent vegetation. While this design choice maintains replication, it may introduce noise into estimates of both LC50 and development time (increasing variance and the size of confidence intervals) due to the fact that directly hatching insects were exposed to volatile terpenoids while eggs, and replaced larvae were not. However, in treatments where substantial replacement was needed due to hatching failure, mortality for replaced larvae was typically high due to the high terpenoid concentrations present (Dataset S1). Replacement was present across all trials and temperature treatments to a similar extent (34%-48% of larvae across the six trials). We interpret these patterns to suggest that the larval period spent feeding on and touching the diet media was more determinative of post-hatching insect outcomes than pre-hatching volatile exposure to terpenoids, but acknowledge this caveat.
Discussion
Terpenes are a common and diverse group of plant secondary metabolites with well-established roles in plant defense (Mithofer and Boland 2012; Padovan et al. 2014). Chemical defense compounds can vary in both abundance and composition in plant tissues depending on a wide range of abiotic factors, including temperature (Bidart-Bouzat and Imeh-Nathaniel 2008), which is also the most influential factor driving the pace of insect development (Lehmann et al. 2020). This study explored the potential for common plant terpenes to inhibit the development and survival of a model generalist herbivore, Vanessa cardui. Our results show that the effects of common terpenes as an anti-herbivore defense vary with temperature and differentially impact different stages of insect development.
The three monoterpenes included in this study (cineole, limonene, and linalool) all displayed a profound ability to prevent eggs from hatching into larvae, an obviously valuable protective effect that would prevent a host plant from being subject to herbivore feeding damage. Egg hatching inhibition increased with dietary terpene concentrations at all temperatures, but this effect was greatest at lower temperatures. Lepidopteran egg deposition has been shown to induce a number of plant defenses responses, including terpene production (Bertea et al. 2020). Our results support existing evidence that terpene concentrations can have a considerable influence on egg hatching (Hilker and Meiners 2011), yet the mechanism for reduced efficacy of the three monoterpenes under increasing temperature is unclear. Given the lack of direct contact between diet and egg, toxicity must be mediated by diffusion of volatiles to the egg surface, a so-called “fumigation effect” reported in several studies (Chaubey 2012). While diffusion into the egg-adjacent air would be expected to increase with temperature and increase the fumigation effect, increasing temperature would also be expected to increase insect metabolism and therefore perhaps the ability of hatching eggs to detoxify terpenes (Lee et al. 2003).
The impact of temperature on the efficacy of monoterpene defenses switches direction in stages beyond egg hatching, with monoterpenes typically displaying higher toxicity to larvae, pupae, and adults at higher temperatures. Linalool displayed the clearest example of this effect, consistently more toxic at higher temperatures across stages from larvae to adult and statistically significant for cumulative adult mortality. The other two monoterpenes (cineole and limonene) show overall the same temperature pattern in toxicity across the three post-hatching life stages, though only certain temperature comparisons for the pupa and adult stages reach statistical significance. However, the sesquiterpene beta-caryophyllene showed no overall effect of temperature on toxicity across any stage, but given that this was the only sesquiterpene tested we cannot draw broad conclusions for this class. Overall, the opposite effects of temperature on egg hatching versus later stage mortality implies that herbivore susceptibility to monoterpene defenses is highly context-dependent, driven by herbivore life stage, ambient temperature, and compound identity.
Similarly to the toxic effects of terpenes, temperature and concentration had distinct and interactive effects on the developmental timing of specimens. As expected for a poikilothermic ectotherm, the pace of development was faster at higher temperatures. However, each terpene had a differential impact on the pace of development between treatments and concentrations. Most terpenes had nonzero delaying effects observed in at least some temperatures, most commonly at the 27°C thermal optimum for development in V. cardui. One mechanism by which terpenes defend plants may be through this extension of development time. Accelerated development time under higher temperatures creates an opportunity for herbivores to both feed more rapidly and reproduce more quickly, and shorter generation time would be expected to increase population size. Both of these effects would escalate the amount of tissue damage experienced by host plants. By delaying development, terpenes may counteract the escalation of herbivore pressure at higher temperatures. Of particular note, even terpenes that exhibited the same absolute magnitude of developmental delaying effect in different temperatures (nondifferent slopes between terpene concentration and development time, Table 2) should properly be considered to have a temperature-dependent delaying effect. This is because the magnitude of delay attributable to terpenes (e.g., 3 days per w/w%) represents a much larger proportion of the total insect development time at warmer temperatures (e.g., 19 days to pupation at 24°C versus 12 days at 30°C), meaning that the same terpene dose inhibits development substantially more at higher temperatures relative to lower temperatures. This may mean that the terpene ‘delaying effect’ is a stronger brake on development at warmer temperatures.
Many plant species have been shown to have seasonal variation in defense investment (Kasey 2010; Karolewski et al. 2013; Mason et al. 2020). Increased production of monoterpenes and sesquiterpenes have been observed to occur in spring and summer which could be a response to either biotic or abiotic conditions (Amaral et al. 2015). We here find that terpenes can be more toxic towards a generalist herbivore at higher temperatures, which suggests during warm seasons plants may be dually defended by increased potency of chemical defenses and their increased production. Variable terpene toxicity was observed across different temperatures, which supports the hypothesis that temperature does impact the efficacy of terpenes as a chemical defense. The shifting of maximum terpene toxicity at different temperatures suggests the existence of terpene production optimums at specific temperatures that would achieve maximum defense against herbivores. All three monoterpenes were less toxic at lower temperatures, so plants would have to produce substantially higher concentrations in colder seasons or climates in order to achieve the same level of defense provided at warmer temperatures. However, herbivore pressure by poikilothermic ectotherms like insects is known to be lower during cooler seasons, such that seasonal trends in terpene production may track herbivore feeding activity either through chemical defense induction (Karban 2011; Züst and Anurag 2017) or seasonal shifts in defense expression arising from either seasonal changes in resource availability or pre-programmed ontogenetic or environmental responses (Schönwitz et al. 1990; Barton and Koricheva 2010; Mason et al. 2020). Terpene production in plants has generally been found to increase under higher temperatures (Ibrahim et al. 2010; Yang et al. 2021), which could mean that plants are able to increase resource allocation to defense under favorable environmental conditions like those typically found during the peak of the growing season (Zvereva and Kozlov 2006), or perhaps that higher terpene production in warmer seasons is a pre-programmed response shaped by natural selection to counteract faster herbivore development.
Plant nutritional quality (as indicated by C:N ratio) has been shown to decrease under elevated CO2 levels at both ambient and elevated temperatures, a phenomenon known as the carbon fertilization effect (Lawler et al. 1996; Drake et al. 2017; Davis et al. 2022). This reduced nutritional quality stimulates herbivores to consume more plant tissue in order to reach their minimum nutritional needs, a phenomenon known as compensatory feeding (Simpson and Simpson 2017). However, no significant fluctuations in nutritional quality have been observed under increased temperatures at ambient CO2 levels (Soares et al. 2019). Meta-analysis indicates that terpene production is largely unaffected by CO2 elevation alone, but is increased when temperature and CO2 are elevated simultaneously (Zvereva and Kozlov 2006). It is possible the combined effect of low nutritional quality and increased terpene production may be enough to counteract the accelerated development of insect herbivores at high temperatures, though this will require careful examination given species- and habitat-specific effects. How future climatic conditions will impact plant responses to herbivory remains largely uncertain at this time, but our results in the context of existing literature indicate that shifts in the production and efficacy of major classes of plant chemical defenses like terpenoids are likely. Previous work indicates that terpene production will likely increase (Holopainen and Gershenzon 2010), and this study suggests that terpene toxicity and developmental effects on herbivores may change as well. If increasing terpenoid production and efficacy imposes stronger selection for insect resistance through detoxification and other mechanisms, herbivore populations may evolve higher tolerance to plant defenses in habitats experiencing substantial warming.
Given that over 40% of global crop production is lost to insect pests annually (IPPC 2021), the finding that plant terpenoids may be more effective (lower LC50) against insect pests at higher temperatures would seem to bode well for agricultural productivity in the face of climate change. However, the benefits of increased terpene toxicity may be counteracted by increased pest voltinism if warming temperatures lengthen the active season while simultaneously speeding insect maturation (Forrest 2016). Plant chemical defenses may be able to slow pest development and interfere with survival to adulthood, but our results suggest that terpene-induced developmental delays are weaker at higher temperatures. Given that insects are expected to experience compensatory feeding as increased warming and atmospheric CO2 increase leaf C:N ratio, this suggests that a given insect pest will consume more plant tissue to meet its dietary needs. Furthermore, increased voltinism equates to an increase in the number of generations per unit absolute time, which increases the potential rate of evolution of populations (Maino et al. 2018). Increased voltinism may also provide the potential for increased insect population size, especially for migratory species that re-establish in a region each active season (Buckley et al. 2017). Increasing population sizes and increased rate of evolution provide increased capacity for insect populations to adapt to new selective pressures (Lande 1988; Debarre and Gandon 2011; Gravel 2016; Jensen et al. 2017), including plant chemical defenses or synthetic pesticides.
Overall, these many individual effects suggest a potential intensification of the plant-herbivore interaction under climate change (Fig. 3). Larger populations of faster-developing insects consume larger amounts of plant tissue, while faster growing plants produce higher quantities of lower-nutrient biomass defended with higher concentrations of more effective carbon-based chemical defenses. While plants might be expected to prevail under such conditions, if large populations of insects are subjected to diets where individuals must ingest larger quantities of more-toxic chemical defenses to meet their minimum nutritional needs, that would constitute a strong selective force for the evolution of resistance to those chemical defenses. While this might result in plants and herbivores reaching a new equilibrium in wild plant populations, this would be unlikely in agricultural settings where crop plants are not typically permitted to evolve in response to herbivore pressure. The evolution of increased insect resistance to plant chemical defenses in natural settings could be a further contributor to agricultural impacts of pest insects, where large increases have been observed thus far under climate change (IPPC 2021). Although this study is limited in scope to the effects of a handful of common plant terpenes in a single cosmopolitan insect herbivore, the patterns of temperature-dependent effects identified raise major questions about the influence of temperature on the efficacy of plant chemical defenses. Temperature-dependent effects of plant chemical defenses should therefore be explicitly considered in the study of seasonality in the fundamental process of herbivory, and the modeling of plant-insect interactions under climate change.
Broader implications of temperature-dependent terpene toxicity under climate change should the results observed for the polyphagous Vanessa cardui in this study (red text) hold for other herbivorous insects. Increased temperatures and atmospheric carbon dioxide concentrations observed under climate change are predicted to have multiple cascading effects on both plants and herbivorous insects. For plants, the increase in growing degree days [1], lengthened growing seasons [2], and the carbon fertilization effect [3] have been demonstrated to result in increased leaf C:N and C:P ratios [4] and increased carbon-based plant defenses [5]. In addition, where other factors like water and nutrient availability are not limiting (*), faster plant growth per unit time [6] is expected to result in increased plant biomass production [7]. For insects, the increase in metabolic rate with increased temperature [8] and the lengthening of active seasons [9] have been demonstrated to result in faster individual development [10] as observed in this study, as well as an increase in the number of generations per year [11], including shifts from univoltine to multivoltine life history in a given geographic region. Where other factors do not limit insect population growth (*), faster development and shorter generation time are expected to result in short-term increases in population size [12], such as within a single growing season. Larger populations of organisms are known to be more able to respond to natural selection [13]. The interactions between plant and insect responses are expected to have multiple effects that influence both the magnitude of herbivory and anti-herbivore plant traits. For insects, the phenomenon of compensatory feeding [14] due to lower leaf nutrient content combined with higher herbivore population sizes [15] would be expected to result in an increase in herbivory pressure on plants. This could be further heightened if hatching rates are higher due to reduced egg stage terpene toxicity as observed in this study, regardless of whether larvae survive to reproduction. For plants, lower nutritional content per unit leaf mass has been demonstrated to reduce herbivore survival and development [16], as have higher concentrations of carbon-based defenses (like terpenes) per unit leaf mass [17]. If the potency of these defenses (toxicity of a given concentration) is also increased by temperature as observed in this study, this will act as a multiplier of the efficacy of plant chemical defense investment. An increase in the magnitude or duration of herbivore pressure would be expected to induce additional production of chemical defenses [18], which may increase concentrations further. Additionally, to the extent that plant growth rate may increase (*) due to the factors described above, this would be expected to increase plant herbivory tolerance through the regrowth of lost or damaged plant parts [19]. Overall, these many individual effects suggest a potential intensification of the plant-herbivore interaction under climate change. References: [1+2] Park et al. 2016; Kukal and Irmak 2018a; Matthews et al. 2018; Piao et al. 2019; [3] McGrath and Lobell 2013; Liang et al. 2016; Terrer et al. 2019; Ueyama et al. 2020; [4] Lincoln et al. 1993; Bezemer and Jones 1998; Gifford et al. 2000; Stiling and Cornelissen 2007; Sardans et al. 2012; Wang et al. 2021; [5+17] Filella et al. 2007; Helmig et al. 2007; Bidart-Bouzat and Imeh-Nathaniel 2008; Ibrahim et al. 2010; Cornelissen 2011; [6+7] Gray and Brady 2016; Park et al. 2016; Kukal and Irmak 2018b; Piao et al. 2019; Babst et al. 2019; Ueyama et al. 2020; [8+9] Cayton et al. 2015; Deutsch et al. 2018; Jactel et al. 2019; Forrest 2016; [10] Colinet et al. 2015; Buckley et al. 2017; Rebaudo and Rabhi 2018; Harvey et al. 2020; [11] Tobin et al. 2008; Altermatt 2010; Ziter et al. 2012; Forrest 2016; [12+15] Colinet et al. 2015; Ziska and McConnell 2016; Tonnang et al. 2017; Harvey et al. 2020; Wagner et al. 2021; Schneider et al. 2022; [13] Lande 1988; Debarre and Gandon 2011; Gravel 2016; Jensen et al. 2017; but see Wood et al. 2016; [14+16] Fajer et al. 1989; Stiling and Cornelissen 2007; Trebicki et al. 2017; Hamann et al. 2021; [18] Agrawal 1998; Agrawal et al. 1999; Underwood 2003; Copolovici et al. 2011; [19] Tiffin 2000; Fornoni 2011; Gray and Brady 2016.
References
Agrawal AA (1998) Induced Responses to Herbivory and Increased Plant Performance. Science 279:1201–1202. https://doi.org/10.1126/science.279.5354.1201
Agrawal AA, Tuzun S, Bent E (1999) Induced Plant Defenses Against Pathogens and Herbivores: Biochemistry, Ecology, and Agriculture. APS Press
Ahmad IM, Waldbauer GP, Friedman S (1989) A defined artificial diet for the larvae of Manduca sexta. Entomol Exp App 53:189–191. https://doi.org/10.1111/j.1570-7458.1989.tb01303.x
Ali MP, Kabir MM, Afrin S, Nowrin F, Haque SS, Haque MM, Hashem A, Tabassum B, Allah EF, Pittendrigh BR (2019) Increased temperature induces leaffolder outbreak in rice field. J Appl Entomol 143:867–874. https://doi.org/10.1111/jen.12652
Altermatt F (2010) Climatic warming increases voltinism in European butterflies and moths. Proc Royal Society B 277:1281–1287. https://doi.org/10.1098/rspb.2009.1910
Amaral LD, Tondolo JS, Schindler B, Silva DT, Pinheiro CG, Longhi SJ, Mallmann CA, Heinzmann BM (2015) Seasonal influence on the essential oil production of Nectandra megapotamica (Spreng.). Mez Brazilian Arch Biol Technol 58:12–21. https://doi.org/10.1590/S1516-8913201502462
Ayres MP, Lombardero MJ (2000) Assessing the consequences of global change for forest disturbance from herbivores and pathogens. Sci Total Environ 262:263–286. https://doi.org/10.1016/S0048-9697(00)00528-3
Babst F, Bouriaud O, Poulter B, Trouet V, Girardin MP, Frank DC (2019) Twentieth century redistribution in climatic drivers of global tree growth. Sci Adv 5:1. https://doi.org/10.1126/sciadv.aat4313
Balandrin MF, Klocke JA (1988) Medicinal, aromatic, and industrial materials from plants. In: Bajaj YPS (eds) Medicinal and Aromatic Plants I. Biotechnology in Agriculture and Forestry, vol 4:3-36. https://doi.org/10.1007/978-3-642-73026-9_1
Bale JS, Masters GJ, Hodkinson ID, Awmack C, Bezemer TM, Brown VK, Butterfield J, Buse A, Coulson JC, Farrar J, Good JE (2002) Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Glob Chang Biol 8:1–16. https://doi.org/10.1046/j.1365-2486.2002.00451.x
Barton K, Koricheva J (2010) The ontogeny of plant defense and herbivory: Characterizing general patterns using meta-analysis. Am Nat 175:481–493. https://doi.org/10.1086/650722
Bates D, Mächler M, Bolker B, Walker S (2014) Fitting linear mixed-effects models using lme4. arXiv Prepr. arXiv1406.5823
Bertea CM, Casacci LP, Bonelli S, Zampollo A, Barbero F (2020) Chemical, physiological and molecular responses of host plants to lepidopteran egg-laying. Front Plant Sci 10:1–7. https://doi.org/10.3389/fpls.2019.01768
Bezemer TM, Jones TH (1998) Plant-insect herbivore interactions in elevated atmospheric CO2: Quantitative analyses and guild effects. Oikos 82:212
Bidart-Bouzat MG, Imeh-Nathaniel A (2008) Global change effects on plant chemical defenses against insect herbivores. J Integr Plant Biol 50:1339–1354. https://doi.org/10.1111/j.1744-7909.2008.00751.x
Blanch J-S, Peñuelas J, Sardans J, Llusià J (2009) Drought, warming and soil fertilization effects on leaf volatile terpene concentrations in Pinus halepensis and Quercus ilex. Acta Physiol Plant 31:207–218. https://doi.org/10.1007/s11738-008-0221-z
Blanchard M, Bowers MD (2020) Critical phenological events affect chemical defense of plant tissues: Iridoid glycosides in a woody shrub. J Chem Eco 46:206–216. https://doi.org/10.1007/s10886-019-01135-8
Buckley LB, Arakaki AJ, Cannistra AF, Kharouba HM, Kingsolver JG (2017) Insect Development, Thermal Plasticity and Fitness Implications in Changing, Seasonal Environments. Integr Comp Biol 57:988–998. https://doi.org/10.1093/icb/icx032
Cayton HL, Haddad NM, Gross K, Diamond SE, Ries L (2015) Do growing degree days predict phenology across butterfly species? Ecology 96:1473–1479. https://doi.org/10.1890/15-0131.1
Celorio-Mancera M (2016) Evolutionary history of host use, rather than plant phylogeny, determines gene expression in a generalist butterfly. BMC Evo Bio 16:1–10. https://doi.org/10.1186/s12862-016-0627-y
Chapman J et al (2015) Long-range seasonal migration in insects: mechanisms, evolutionary drivers and ecological consequences. Ecol Lett 18:287–302
Chaubey MK (2012) Acute, lethal and synergistic effects of some terpenes against Tribolium castaneum Herbst (Coleoptera: Tenebrionidae). Ecol Balk 4:53–62
Chippendale GM (1982) Insect diapause, the seasonal synchronization of life cycles, and management strategies. Entomol Exp et Appl 31:24–35. https://doi.org/10.1111/j.1570-7458.1982.tb03116.x
Coats JR, Karr LL, Drewes CD (1991) Toxicity and neurotoxic effects of monoterpenoids in insects and earthworms. In: Naturally occurring pest bioregulators. American Chemical Society, Washington, DC, pp 305–316
Colinet H, Sinclair BJ, Vernon P, Renault D (2015) Insects in Fluctuating Thermal Environments. Annu Rev Entomol 60:123–140. https://doi.org/10.1146/annurev-ento-010814-021017
Connahs H, Rhen T, Simmons RB (2016) Transcriptome analysis of the painted lady butterfly, Vanessa cardui during wing color pattern development. BMC Genomics 17:1–16. https://doi.org/10.1186/s12864-016-2586-5
Copolovici L, Kännaste A, Remmel T, Vislap V, Niinemets Ü (2011) Volatile Emissions from Alnus glutionosa Induced by Herbivory are Quantitatively Related to the Extent of Damage. J Chem Ecol 37:18–28. https://doi.org/10.1007/s10886-010-9897-9
Cornelissen T (2011) Climate change and its effects on terrestrial insects and herbivory patterns. Neotrop Entomol 40:155–163. https://doi.org/10.1590/s1519-566x2011000200001
Davis EC, Sohngen B, Lewis DJ (2022) The effect of carbon fertilization on naturally regenerated and planted US forests. Nature Comm 13:5490
Debarre F, Gandon S (2011) Evolution in Heterogeneous Environments: Between Soft and Hard Selection. Am Nat 177:E84–E97. https://doi.org/10.1086/658178
Deutsch CA et al (2018) Increase in crop losses to insect pests in a warming climate. Science 361:916–919. https://doi.org/10.1126/science.aat3466
Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7:1085–1097. https://doi.org/10.1105/tpc.7.7.1085
Drake BL, Hanson DT, Lowrey TK, Sharp ZD (2017) The carbon fertilization effect over a century of anthropogenic CO2 emissions: higher intracellular CO2 and more drought resistance among invasive and native grass species contrasts with increased water use efficiency for woody plants in the US Southwest. Glob Change Biol 23:782–792. https://doi.org/10.1111/gcb.13449
Ehrlich PR, Raven PH (1964) Butterflies and plants: a study in coevolution. Evolution 18(4):586–608. https://doi.org/10.2307/2406212
Fajer ED, Bowers MD, Bazzaz FA (1989) The effects of enriched carbon dioxide atmospheres on plant-insect herbivore interactions. Science 243:1198–1200
Feeny P (1970) Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 51:565–581. https://doi.org/10.2307/1934037
Filella I, Wilkinson MJ, Llusià J, Hewitt CN, Peñuelas J (2007) Volatile organic compounds emissions in Norway spruce (Picea abies) in response to temperature changes. Physiol Plant 130:58–66
Fornoni J (2011) Ecological and evolutionary implications of plant tolerance to herbivory. Funct Ecol 25:399–407. https://doi.org/10.1111/j.1365-2435.2010.01805.x
Forrest JRK (2016) Complex responses of insect phenology to climate change. Curr Opin Insect Sci 17:49–54. https://doi.org/10.1016/j.cois.2016.07.002
Fuentealba A et al (2017) How does synchrony with host plant affect the performance of an outbreaking insect defoliator? Oecologia 184:847–857. https://doi.org/10.1007/s00442-017-3914-4
Gershenzon J, Dudareva N (2012) The function of terpene natural products in the natural world. Nat Chem Bio 7:408–414. https://doi.org/10.1038/nchembio.2007.5
Gifford RM, Barrett DJ, Lutze JL (2000) The effects of elevated CO2 on the C:N and C:P mass ratios of plant tissues. Plant Soil 224:1–14. https://doi.org/10.1023/A:1004790612630
Goehring L, Oberhauser KS (2002) Effects of photoperiod, temperature, and host plant age on induction of reproductive diapause and development time in Danaus plexippus. Ecol Entomol 24:674–685. https://doi.org/10.1046/j.1365-2311.2002.00454.x
Gols R et al (2018) Seasonal and herbivore-induced dynamics of foliar glucosinolates in wild cabbage (Brassica oleracea). Chemoecology 28:77–89. https://doi.org/10.1007/s00049-018-0258-4
Gravel S (2016) When Is Selection Effective? Genetics 203:451–462. https://doi.org/10.1534/genetics.115.184630
Gray SB, Brady SM (2016) Plant developmental responses to climate change. Dev Biol 419:64–77. https://doi.org/10.1016/j.ydbio.2016.07.023
Hahn DA, Denlinger DL (2011) Energetics of insect diapause. Annu Rev Entomol 56:103–121. https://doi.org/10.1146/annurev-ento-112408-085436
Hamann E, Blevins C, Franks SJ, Jameel MI, Anderson JT (2021) Climate change alters plant–herbivore interactions. New Phyt 229:1894–1910. https://doi.org/10.1111/nph.17036
Harvey JA, Heinen R, Gols R, Thakur MP (2020) Climate change-mediated temperature extremes and insects: From outbreaks to breakdowns. Glob Chang Biol 26:6685–6701. https://doi.org/10.1111/gcb.15377
Helmig D, Ortega J, Duhl T, Tanner D, Guenther A, Harley P, Wiedinmyer D, Milford J, Sakulyanontvittaya T (2007) Sesquiterpene emissions from pine trees-identifications, emission rates and flux estimates for the contiguous united states. Environ Sci Technol 4:1545–1553. https://doi.org/10.1021/es0618907
Hilker M, Meiners T (2011) Plants and insect eggs: How do they affect each other? Phytochemistry 72:1612–1623. https://doi.org/10.1016/j.phytochem.2011.02.018
Holopainen JK, Gershenzon J (2010) Multiple stress factors and the emission of plant VOCs. Trends Plant Sci 15:176–184. https://doi.org/10.1016/j.tplants.2010.01.006
Howe GA, Jander G (2008) Plant immunity to insect herbivores. Annu Rev Plant Biol 59:41–66. https://doi.org/10.1146/annurev.arplant.59.032607.092825
Hu G, Stefanescu C, Oliver TH, Roy DB, Brereton T, Van Swaay C, Reynolds DR, Chapman JW (2021) Environmental drivers of annual population fluctuations in a trans-Saharan insect migrant. PNAS 118:e2102762118. https://doi.org/10.1073/pnas.2102762118
Hunter MD (1992) A variable insect–plant interaction: the relationship between tree budburst phenology and population levels of insect herbivores among trees. Ecol Entomol 17:91–95. https://doi.org/10.1111/j.1365-2311.1992.tb01046.x
Ibrahim MA, Mäenpää M, Hassinen V, Kontunen-Soppela S, Malec L, Rousi M, Pietikäinen L (2010) Elevation of night-time temperature increases terpenoid emissions from Betula pendula and Populus tremula. J Exp Bot 61:1583–1595. https://doi.org/10.1093/jxb/erq034
IPPC Secretariat (2021) Scientific Review of the Impact of Climate Change on Plant Pests: A Global Challenge to Prevent and Mitigate Plant-Pest Risks in Agriculture, Forestry and Ecosystems. FAO
Jactel H, Koricheva J, Castagneyrol B (2019) Responses of forest insect pests to climate change: not so simple. Curr Opin Insect Sci 35:103–108. https://doi.org/10.1016/j.cois.2019.07.010
Jensen K, Kristensen TN, Heckmann L, Sørensen JG (2017) Breeding and maintaining high-quality insects. Insects as food and feed: from production to consumption. Wageningen Academic Publishers, Wageningen, Netherlands:175-198
Karban R (2011) The ecology and evolution of induced resistance against herbivores. Funct Ecol 25:339–347. https://doi.org/10.1111/j.1365-2435.2010.01789.x
Karolewski P, Giertych MJ, Żmuda M, Jagodziński AM, Oleksyn J (2013) Season and light affect constitutive defenses of understory shrub species against folivorous insects. Acta Oecologica 53:19–32. https://doi.org/10.1016/j.actao.2013.08.004
Kasey KJ (2010) The ontogeny of plant defense and herbivory: characterizing general patterns using meta‐analysis. Am Nat 175:481–493. https://doi.org/10.1086/650722
Kelly L, Debinski DM (1999) Effects of larval food-limitation on Vanessa cardui Linnaeus (Lepidoptera: Nymphalidae). Am Midl Nat 141:315–322
King DJ, Gleadow RM, Woodrow IE (2006) The accumulation of terpenoid oils does not incur a growth cost in Eucalyptus polybractea seedlings. Funct Plant Biol 33:497–505. https://doi.org/10.1071/FP05304
Kleiner KW (1989) Sources of variation in oak leaf quality as food for the gypsy moth: implications for forest stand susceptibility. Dissertation, Pennsylvania State University
Kopaczyk JM, Warguła J, Jelonek T (2020) The variability of terpenes in conifers under developmental and environmental stimuli. Environ Exp Botany 180:104197. https://doi.org/10.1016/j.envexpbot.2020.104197
Kukal MS, Irmak S (2018a) U.S. Agro-Climate in 20th Century: Growing Degree Days, First and Last Frost, Growing Season Length, and Impacts on Crop Yields. Sci Rep 8:6977. https://doi.org/10.1038/s41598-018-25212-2
Kukal MS, Irmak S (2018b) Climate-driven crop yield and yield variability and climate change impacts on the US Great Plains agricultural production. Sci Rep 8:1–18
Kumbasli M, Bauce E (2013) Spruce budworm biological and nutritional performance responses to varying levels of monoterpenes. iForest 6:310–314. https://doi.org/10.3832/ifor0956-006
Lande R (1988) Genetics and demography in biological conservation. Science 241:1455–1460
Lawler IR et al (1996) The effects of elevated CO2 atmospheres on the nutritional quality of Eucalyptus foliage and its interaction with soil nutrient and light availability. Oecologia 109(1):59–68
Lee S, Peterson CJ, Coats JR (2003) Fumigation toxicity of monoterpenoids to several stored product insects. J Stored Prod Res 39:77–85. https://doi.org/10.1016/s0022474x(02)00020-6
Lehmann P, Ammunét T, Barton M, Battisti A, Eigenbrode SD, Jepsen JU, Kalinkat G, Neuvonen S, Niemelä P, Terblanche JS, Økland B, Björkman C (2020) Complex responses of global insect pests to climate warming. Front Ecol Environ 18:141–150. https://doi.org/10.1002/fee.2160
Li Y, Hu L, Romeis J, Wang Y, Han L, Chen X, Peng Y (2014) Use of an artificial diet system to study the toxicity of gut active insecticidal compounds on larvae of the green lacewing Chrysoperla sinica. Biol Control 69:45–51. https://doi.org/10.1016/j.biocontrol.2013.10.017
Liang J, Qi X, Souza L, Luo Y (2016) Processes regulating progressive nitrogen limitation under elevated carbon dioxide: a meta-analysis. Biogeosciences 13:2689–2699. https://doi.org/10.5194/bg-13-2689-2016
Lincoln DE, Fajer ED, Johnson RH (1993) Plant-Insect Herbivore Interactions in Elevated CO2 Environments. Trends Ecol Evol 8:64–68. https://doi.org/10.1016/0169-5347(93)90161-H
Lüdecke D, Ben-Shachar M, Patil I, Waggoner P, Makowski D (2021) performance: An R Package for Assessment, Comparison and Testing of Statistical Models. J Open Source Software 60:3139. https://doi.org/10.21105/joss.03139
Maino JL, Umina PA, Hoffmann AA (2018) Climate contributes to the evolution of pesticide resistance. Glob Ecol Biogeogr 27:223–232. https://doi.org/10.1111/geb.12692
Marini L, Økland B, Jönsson AM, Bentz B, Carroll A, Forster B, Grégoire JC, Hurling R, Nageleisen LM, Netherer S, Ravn HP (2017) Climate drivers of bark beetle outbreak dynamics in Norway spruce forests. Ecography 40:1426–1435. https://doi.org/10.1111/ecog.02769
Mason CM, LaScaleia MC, De La Pascua DR, Monroe JG, Goolsby EW (2020) Learning from dynamic traits: Seasonal shifts yield insights into ecophysiological trade-offs across scales from macroevolutionary to intraindividual. Int J Plant Sci 181:88–102. https://doi.org/10.1086/706238
Matthews SN, Iverson LR, Peters MP, Prasad AM (2018) Assessing potential climate change pressures across the conterminous United States: mapping plant hardiness zones, heat zones, growing degree days, and cumulative drought severity throughout this century. RMAP-NRS-9. Newtown Square, PA: US Department of Agriculture, Forest Service, Northern Research Station. 31 p. 9:1-31. doi:https://doi.org/10.2737/NRS-RMAP-9
McGrath JM, Lobell DB (2013) Regional disparities in the CO 2 fertilization effect and implications for crop yields. Environ Res Lett 8:014054. https://doi.org/10.1088/1748-9326/8/1/014054
Meineke EK, Classen AT, Sanders NJ, Jonathan Davies T (2019) Herbarium specimens reveal increasing herbivory over the past century. Ecology 107:105–117. https://doi.org/10.1111/1365-2745.13057
Mewalal R, Rai DK, Kainer D et al (2017) Plant-Derived Terpenes: A Feedstock for Specialty Biofuels. Trends Biotechnol 35:227–240. https://doi.org/10.1016/j.tibtech.2016.08.003
Mithöfer A, Boland W (2012) Plant defense against herbivores: chemical aspects. Ann Rev Plant Biol 63:431–450. https://doi.org/10.1146/annurev-arplant-042110-103854
Padovan A, Keszei A, Külheim C, Foley WJ (2014) The evolution of foliar terpene diversity in Myrtaceae. Phytochem Rev 13:695–716. https://doi.org/10.1007/s11101-013-9331-3
Pakyari H, Amir-Maafi M, Moghadamfar Z, Zalucki M (2019) Estimating development and temperature thresholds of Ephestia kuehniella: toward improving a mass production system. Bull Entomol Res 109:435–442. https://doi.org/10.1017/S0007485318000718
Park T et al (2016) Changes in growing season duration and productivity of northern vegetation inferred from long-term remote sensing data. Environ Res Lett 11:084001. https://doi.org/10.1088/1748-9326/11/8/084001
Piao S et al (2019) Plant phenology and global climate change: Current progresses and challenges. Glob Change Bio 25:1922–1940. https://doi.org/10.1111/gcb.14619
Pichersky E, Raguso RA (2018) Why do plants produce so many terpenoid compounds? New Phyt 220:692–702. https://doi.org/10.1111/nph.14178
Porter JH, Parry ML, Carter TR (1991) The potential effects of climatic change on agricultural insect pests. Agric For Meteorol 57:221–240. https://doi.org/10.1016/0168-1923(91)90088-8
Poston FL, Hammond RB, Pedigo LP (1977) Growth and development of the painted lady on soybeans (Lepidoptera:Nymphalidae). J Kans Entomol Soc 50:31–36
Rebaudo F, Rabhi VB (2018) Modeling temperature-dependent development rate and phenology in insects: review of major developments, challenges, and future directions. Entomol Exp Appl 166:607–617. https://doi.org/10.1111/eea.12693
Sardans J, Rivas-Ubach A, Peñuelas J (2012) The C:N:P stoichiometry of organisms and ecosystems in a changing world: A review and perspectives. Perspect Plant Ecol Evol and Syst 14:33–47. https://doi.org/10.1016/j.ppees.2011.08.002
Saunier A, Ormeño E, Moja S et al (2022) Lavender sensitivity to water stress: Comparison between eleven varieties across two phenological stages. Industrial Crops and Products 177:114531. https://doi.org/10.1016/j.indcrop.2022.114531
Schneider L, Rebetez M, Rasmann S (2022) The effect of climate change on invasive crop pests across biomes. Curr Opin Insect Sci 50:100895. https://doi.org/10.1016/j.cois.2022.100895
Schönwitz R et al (1990) Seasonal variation in the monoterpenes in needles of Picea abies (L.) Karst. Trees 4:34–40
Simpson SJ, Simpson SL (2017) The mechanisms of nutritional compensation by phytophagousinsects. Insect-plant interactions. pp 111-160
Soares JC, Santos CS, Carvalho SMP, Pintada MM, Vasconcelos MW (2019) Preserving the nutritional quality of crop plants under a changing climate: importance and strategies. Plant Soil 443:1–26. https://doi.org/10.1007/s11104-019-04229-0
Song X, Wen X, He J et al (2019) Phytochemical components and biological activities of Artemisia argyi. J Functional Foods 52:648–662. https://doi.org/10.1016/j.jff.2018.11.029
Sørensen JG, Addison MF, Terblanche JS (2012) Mass-rearing of insects for pest management: Challenges, synergies and advances from evolutionary physiology. Crop Prot 38:87–94. https://doi.org/10.1016/j.cropro.2012.03.023
Stiling P, Cornelissen T (2007) How does elevated carbon dioxide (CO2) affect plant–herbivore interactions? A field experiment and meta-analysis of CO2-mediated changes on plant chemistry and herbivore performance. Glob Change Biol 13:1823–1842. https://doi.org/10.1111/j.1365-2486.2007.01392.x
Stoeckli S, Hirschi M, Spirig C, Calanca P, Rotach MW, Samietz (2012) Impact of climate change on voltinism and prospective diapause induction of a global pest insect – Cydia pomonella (L.). PLoS ONE. https://doi.org/10.1371/journal.pone.0035723
Teder T (2020) Phenological responses to climate warming in temperate moths and butterflies: species traits predict future changes in voltinism. Oikos 129:1051–1060. https://doi.org/10.1111/oik.07119
Terrer C et al (2019) Nitrogen and phosphorus constrain the CO2 fertilization of global plant biomass. Nat Clim Change 9:684–689. https://doi.org/10.1038/s41558-019-0545-2
Tiffin P (2000) Mechanisms of tolerance to herbivore damage: what do we know? Evol Ecol 14:523–536. https://doi.org/10.1023/A:1010881317261
Tissier A, Ziegler J, Vogt T (2014) Specialized plant metabolites: diversity and biosynthesis. In: Krauss GJ, Nies DH (eds) Ecological biochemistry: environmental and interspecies interactions. Wiley, Weinheim, pp 14–37. https://doi.org/10.1002/9783527686063.ch2
Tobin PC, Nagarkatti S, Loeb G, Saunders MC (2008) Historical and projected interactions between climate change and insect voltinism in a multivoltine species. Glob Change Biol 14:951–957
Tonnang HEZ et al (2017) Advances in crop insect modelling methods—Towards a whole system approach. Ecol Modell 354:88–103. https://doi.org/10.1016/j.ecolmodel.2017.03.015
Trębicki P, Dáder B, Vassiliadis S, Fereres A (2017) Insect–plant–pathogen interactions as shaped by future climate: effects on biology, distribution, and implications for agriculture. Insect Sci 24:975–989. https://doi.org/10.1111/1744-7917.12531
Ueyama M et al (2020) Inferring CO2 fertilization effect based on global monitoring land-atmosphere exchange with a theoretical model. Environ Res Lett 15:084009. https://doi.org/10.1088/1748-9326/ab79e5
Underwood N (2003) Density dependence in induced plant resistance to herbivore damage: threshold, strength and genetic variation. Oikos 89:295–300. https://doi.org/10.1034/j.1600-0706.2000.890210.x
Venables WN, Ripley BD (2002) Modern applied statistics with S fourth edition. World
Wagner DL, Grames EM, Forister ML, Berenbaum MR, Stopak D (2021) Insect decline in the Anthropocene: Death by a thousand cuts. PNAS 118:e2023989118. https://doi.org/10.1073/pnas.2023989118
Wang C, Sun Y, Chen HYH, Ruan H (2021) Effects of elevated CO2 on the C:N stoichiometry of plants, soils, and microorganisms in terrestrial ecosystems. Catena 201:105219. https://doi.org/10.1016/j.catena.2021.105219
Williams CB (1970) Migrations of the painted lady butterfly, Vanessa cardui (Nymphalidae), with special reference to North America. J Lepid Soc 24:157–173
Wint W (1983) The role of alternative host-plant species in the life of a polyphagous moth, Operophtera brumata (Lepidoptera: Geometridae). J Anim Ecol 52:439–450. https://doi.org/10.2307/4564
Wood JLA, Yates MC, Fraser DJ (2016) Are heritability and selection related to population size in nature? Meta-analysis and conservation implications. Evol Appl 9:640–657. https://doi.org/10.1111/eva.12375
Yang W et al (2021) Review on plant terpenoid emissions worldwide and in China. Sci Total Environ 787:147454
Yukawa J, Akimoto K (2006) Influence of synchronization between adult emergence and host plant phenology on the population density of Pseudasphondylia neolitseae (Diptera: Cecidomyiidae) inducing leaf galls on Neolitsea sericea (Lauraceae). Popul Ecol 48:13–21. https://doi.org/10.1007/s10144-005-0233-0
Ziska LH, McConnell LL (2016) Climate Change, Carbon Dioxide, and Pest Biology: Monitor, Mitigate, Manage. J Agric Food Chem 64:6–12. https://doi.org/10.1021/jf506101h
Ziter C, Robinson EA, Newman JA (2012) Climate Change and Voltinism in Californian Insect Pest Species: Sensitivity to Location, Scenario and Climate Model Choice. Glob Change Biol 18(9):2771–2780
Züst T, Anurag AA (2017) Trade-offs between plant growth and defense against insect herbivory: an emerging mechanistic synthesis. Annu Rev Plant Bio 68:513–534
Zvereva EL, Kozlov MV (2006) Consequences of simultaneous elevation of carbon dioxide and temperature for plant-herbivore interactions: a metaanalysis. Glob Chang Biol 12:27–41. https://doi.org/10.1111/j.1365-2486.2005.01086.x
Acknowledgements
The authors thank Michelle Tepetate for her early assistance with the project and Jordan Dowell for his guidance and insight. We would also like to thank Samantha Mason and Dr. Kenneth Fedorka for their assistance in obtaining incubators used in this study.
Data availability
Datasets used in this study are included in the supplemental information and will be deposited in the Dryad Digital Repository upon manuscript acceptance.
Funding
This work was supported by startup funds granted to Chase M. Mason by the University of Central Florida
Author information
Authors and Affiliations
Contributions
MRI, CMM, and SL conceived and designed the experiments. MRI, HS, and MU performed the experiments. EWG and MRI led data analysis. MRI, EWG, and CMM wrote the manuscript with feedback from the other authors.
Corresponding author
Ethics declarations
Ethics approval
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no relevant financial or non-financial interests to disclose.
Supplementary Information
Data 1
Full data on Vanessa cardui hatching failure and larval, pupa, and adult mortality presented as counts and proportions. Run represents individual trial runs, and temperature is presented in Celsius. Concentration of each terpene is presented in w/w percentage as prepared at the start of each trial. See full text for methods details. (CSV 12 kb)
Data 2
Full data on Vanessa cardui development time from hatching to pupation, and from hatching to eclosure (emergence from pupa). Run represents individual trial runs, and temperature is presented in Celsius. Concentration of each terpene is presented in w/w percentage as prepared at the start of each trial. See full text for methods details. (CSV 39 kb)
Data 3
Full data on Vanessa cardui adult dry mass. Run represents individual trial runs, and temperature is presented in Celsius. Concentration of each terpene is presented in w/w percentage as prepared at the start of each trial. See full text for methods details. (CSV 29 kb)
ESM 4
(DOCX 4357 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Irving, M.R., Goolsby, E.W., Stanford, H. et al. Temperature alters the toxicological impacts of plant terpenoids on the polyphagous model herbivore Vanessa cardui. J Chem Ecol 49, 666–680 (2023). https://doi.org/10.1007/s10886-023-01449-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-023-01449-8