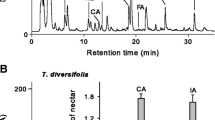
Abstract
Plant secondary metabolites that defend leaves from herbivores also occur in floral nectar. While specialist herbivores often have adaptations providing resistance to these compounds in leaves, many social insect pollinators are generalists, and therefore are not expected to be as resistant to such compounds. The milkweeds, Asclepias spp., contain toxic cardenolides in all tissues including floral nectar. We compared the concentrations and identities of cardenolides between tissues of the North American common milkweed Asclepias syriaca, and then studied the effect of the predominant cardenolide in nectar, glycosylated aspecioside, on an abundant pollinator. We show that a generalist bumblebee, Bombus impatiens, a common pollinator in eastern North America, consumes less nectar with experimental addition of ouabain (a standard cardenolide derived from Apocynacid plants native to east Africa) but not with addition of glycosylated aspecioside from milkweeds. At a concentration matching that of the maximum in the natural range, both cardenolides reduced activity levels of bees after four days of consumption, demonstrating toxicity despite variation in behavioral deterrence (i.e., consumption). In vitro enzymatic assays of Na+/K+-ATPase, the target site of cardenolides, showed lower toxicity of the milkweed cardenolide than ouabain for B. impatiens, indicating that the lower deterrence may be due to greater tolerance to glycosylated aspecioside. In contrast, there was no difference between the two cardenolides in toxicity to the Na+/K+-ATPase from a control insect, the fruit fly Drosophila melanogaster. Accordingly, this work reveals that even generalist pollinators such as B. impatiens may have adaptations to reduce the toxicity of specific plant secondary metabolites that occur in nectar, despite visiting flowers from a wide variety of plants over the colony’s lifespan.




Similar content being viewed by others
Data availability
All of the raw data is available as supplementary material.
References
Adler LS (2000) The ecological significance of toxic nectar. Oikos 91:409–420
Adler LS, Wink M, Distl M, Lentz AJ (2006) Leaf herbivory and nutrients increase nectar alkaloids. Ecol Lett 9:960–967
Agrawal AA et al (2022) Functional evidence supports adaptive plant chemical defense along a geographical cline. Proc Natl Acad Sci USA 119:e2205073119
Agrawal AA, Petschenka G, Bingham RA, Weber MG, Rasmann S (2012) Toxic cardenolides: chemical ecology and coevolution of specialized plant–herbivore interactions. New Phytol 194:28–45
Baracchi D, Brown MJ, Chittka L (2015) Behavioural evidence for self-medication in bumblebees? F1000Res 4:73
Bates D, Mächler M, Bolker B, Walker S (2014) Fitting linear mixed-effects models using lme4. arXiv:14065823
Bennett RN, Wallsgrove RM (1994) Secondary metabolites in plant defence mechanisms. New Phytol 127:617–633
Betz RF, Struven RD, Wall JE, Heitler FB (1994) Insect pollinators of 12 milkweed (Asclepias) species. In: Wickett RG, Lewis PD, Woodliffe A, Pratt P (eds) Proceedings of the Thirteenth North American Prairie Conference: Spirit of the Land, our Prairie Legacy. Department of Parks & Recreation, Windsor, pp 45–60
Brower LP, McEvoy PB, Williamson KL, Flannery MA (1972) Variation in cardiac glycoside content of monarch butterflies from natural populations in eastern North America. Science 177:426–429
Cane JH, Gardner DR, Weber M (2020) Neurotoxic alkaloid in pollen and nectar excludes generalist bees from foraging at death-camas, Toxicoscordion paniculatum (Melanthiaceae). Biol J Linn Soc 131:927–935
Detzel A, Wink M (1993) Attraction, deterrence or intoxication of bees (Apis mellifera) by plant allelochemicals. Chemoecology 4:8–18
Dobler S, Dalla S, Wagschal V, Agrawal AA (2012) Community-wide convergent evolution in insect adaptation to toxic cardenolides by substitutions in the Na, K-ATPase. Proc Natl Acad Sci USA 109:13040–13045
Fishbein M, Venable DL (1996) Diversity and temporal change in the effective pollinators of Asclepias tuberosa. Ecology 77:1061–1073
Fontaine C, Thébault E, Dajoz I (2009) Are insect pollinators more generalist than insect herbivores? Proc R Soc B: Biol Sci 276:3027–3033
Gervais A, Courtois È, Fournier V, Bélisle M (2020) Landscape composition and local floral resources influence foraging behavior but not the size of Bombus impatiens Cresson (Hymenoptera: Apidae) workers. PLoS ONE 15:e0234498
Gosselin M, Michez D, Vanderplanck M, Roelants D, Glauser G, Rasmont P (2013) Does Aconitum septentrionale chemically protect floral rewards to the advantage of specialist bumblebees? Ecol Entomol 38:400–407
Hartmann T, Theuring C, Schmidt J, Rahier M, Pasteels JM (1999) Biochemical strategy of sequestration of pyrrolizidine alkaloids by adults and larvae of chrysomelid leaf beetles. J Insect Physiol 45:1085–1095
Heinrich B (1976) Resource partitioning among some eusocial insects: bumblebees. Ecology 57:874–889
Hoch JH (1961) A survey of cardiac glycosides and genins. University of South Carolina Press, Columbia, SC USA
Inouye DW (1978) Resource partitioning in bumblebees: experimental studies of foraging behavior. Ecology 59:672–678
Ivey CT, Martinez P, Wyatt R (2003) Variation in pollinator effectiveness in swamp milkweed, Asclepias incarnata (Apocynaceae). Am J Bot 90:214–225
Jacobsen DJ, Raguso RA (2018) Lingering effects of herbivory and plant defenses on pollinators. Curr Biol 28:1164–1169
Janzen DH (1977) Why don’t ants visit flowers. Biotropica 9:252
Jennersten O, Morse DH (1991) The quality of pollination by diurnal and nocturnal insects visiting common milkweed, Asclepias syriaca. Am Midl Nat 125:18–28
Jones PL, Agrawal AA (2016) Consequences of toxic secondary compounds in nectar for mutualist bees and antagonist butterflies. Ecology 97:2570–2579
Karageorgi M et al (2019) Genome editing retraces the evolution of toxin resistance in the monarch butterfly. Nature 574:409–412
Kephart SR (1983) The partitioning of pollinators among three species of Asclepias. Ecology 64:120–133
Kessler A, Halitschke R (2009) Testing the potential for conflicting selection on floral chemical traits by pollinators and herbivores: predictions and case study. Funct Ecol 23:901–912
Kessler D, Baldwin IT (2007) Making sense of nectar scents: the effects of nectar secondary metabolites on floral visitors of Nicotiana attenuata. Plant J 49:840–854
Kessler D, Gase K, Baldwin IT (2008) Field experiments with transformed plants reveal the sense of floral scents. Science 321:1200–1202
Laverty TM, Plowright R (1988) Flower handling by bumblebees: a comparison of specialists and generalists. Anim Behav 36:733–740
Lenth R, Singmann H, Love J, Buerkner P, Herve M (2020) Emmeans: estimated marginal means. R package version 1.4. 4. Am Stat 34(4):216–221
López-Goldar X, Hastings A, Züst T, Agrawal A (2022) Evidence for tissue-specific defence-offence interactions between milkweed and its community of specialized herbivores. Mol Ecol 31:3254–3265
Malcolm SB (1991) Cardenolide-mediated interactions between plants and herbivores Herbivores: their interactions with secondary plant metabolites. In: Rosenthal GA, Berenbaum MR (eds) Herbivores Their Interactions with Secondary Plant Metabolites, 2nd edn. Academic Press Inc, San Deigo, pp 251–296
Manson JS, Otterstatter MC, Thomson JD (2010) Consumption of a nectar alkaloid reduces pathogen load in bumble bees. Oecologia 162:81–89
Manson JS, Rasmann S, Halitschke R, Thomson JD, Agrawal AA (2012) Cardenolides in nectar may be more than a consequence of allocation to other plant parts: a phylogenetic study of Asclepias. Funct Ecol 26:1100–1110
Morse DH, Fritz RS (1983) Contributions of diurnal and nocturnal insects to the pollination of common milkweed (Asclepias syriaca L.) in a pollen-limited system. Oecologia 60:190–197
Oksanen J, Simpson G, Blanchet F, Kindt R, Legendre P, Minchin P, O'Hara R, Solymos P, Stevens M, Szoecs E, Wagner H, Barbour M, Bedward M, Bolker B, Borcard D, Carvalho G, Chirico M, De Caceres M, Durand S, Evangelista H, FitzJohn R, Friendly M, Furneaux B, Hannigan G, Hill M, Lahti L, McGlinn D, Ouellette M, Ribeiro Cunha E, Smith T, Stier A, Ter Braak C, Weedon J (2013) vegan: Community Ecology Package. R package version 2.6-2. https://cran.r-project.org/web/packages/vegan/index.html
Paula S, Tabet MR, Ball WJ (2005) Interactions between cardiac glycosides and sodium/potassium-ATPase: three-dimensional structure− activity relationship models for ligand binding to the E2-pi form of the enzyme versus activity inhibition. Biochemistry 44:498–510
Petschenka G, Fandrich S, Sander N, Wagschal V, Boppré M, Dobler S (2013) Stepwise evolution of resistance to toxic cardenolides via genetic substitutions in the Na+/K+-ATPase of milkweed butterflies (Lepidoptera: Danaini). Evolution 67:2753–2761
Petschenka G, Fei CS, Araya JJ, Schröder S, Timmermann BN, Agrawal AA (2018) Relative selectivity of plant cardenolides for Na+/K+-ATPases from the monarch butterfly and non-resistant insects. Front Plant Sci 9:1424
Petschenka G, Offe JK, Dobler S (2012) Physiological screening for target site insensitivity and localization of Na+/K+-ATPase in cardenolide-adapted Lepidoptera. J Insect Physiol 58:607–612
Petschenka G, Wagschal V, von Tschirnhaus M, Donath A, Dobler S (2017) Convergently evolved toxic secondary metabolites in plants drive the parallel molecular evolution of insect resistance. Am Nat 190:S29–S43
Pinheiro J, Bates D, DebRoy S, Sarkar D, Team RC (2007) Linear and nonlinear mixed effects models. R package version 3:1–89. https://cran.r-project.org/web/packages/nlme/index.html
Pluskal T, Castillo S, Villar-Briones A, Orešič M (2010) MZmine 2: modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinformatics 11:1–11
Ranta E, Lundberg H (1980) Resource partitioning in bumblebees: the significance of differences in proboscis length. Oikos 35:298–302
Rasmann S, Agrawal AA (2011) Latitudinal patterns in plant defense: evolution of cardenolides, their toxicity and induction following herbivory. Ecol Lett 14:476–483
Rhoades DF, Bergdahl JC (1981) Adaptive significance of toxic nectar. Am Nat 117:798–803
Richardson LL et al (2015) Secondary metabolites in floral nectar reduce parasite infections in bumblebees. Proc R Soc B: Biol Sci 282:20142471
Richardson LL, Bowers MD, Irwin RE (2016) Nectar chemistry mediates the behavior of parasitized bees: consequences for plant fitness. Ecology 97:325–337
Schmitt A, Roy R, Carter CJ (2021) Nectar antimicrobial compounds and their potential effects on pollinators. Curr Opin Insect Sci 44:55–63
Stevenson PC (2020) For antagonists and mutualists: the paradox of insect toxic secondary metabolites in nectar and pollen. Phytochem Rev 19:603–614
Stevenson PC, Nicolson SW, Wright GA (2017) Plant secondary metabolites in nectar: impacts on pollinators and ecological functions. Funct Ecol 31:65–75
Thøstesen AM, Olesen JM (1996) Pollen removal and deposition by specialist and generalist bumblebees in Aconitum septentrionale. Oikos 77:77–84
Tiedeken EJ, Stout JC, Stevenson PC, Wright GA (2014) Bumblebees are not deterred by ecologically relevant concentrations of nectar toxins. J Exp Biol 217:1620–1625
Villalona E, Ezray BD, Laveaga E, Agrawal AA, Ali JG, Hines HM (2020) The role of toxic nectar secondary compounds in driving differential bumble bee preferences for milkweed flowers. Oecologia 193:619–630
Wright G et al (2013) Caffeine in floral nectar enhances a pollinator’s memory of reward. Science 339:1202–1204
Acknowledgements
This research was funded by NSF grant #1907375 to AAA and PLJ and by Bowdoin College funding to PLJ.
Funding
This research was funded by NSF grant #1907375 to AAA and PLJ and by Bowdoin College funding to PLJ.
Author information
Authors and Affiliations
Contributions
PLJ, AAA, and KRM designed the experiment. KRM conducted the bee consumption and activity level experiments. APH collected milkweed tissues. CD conducted the mass spectrometry. APH conducted the enzyme assays. SVP analyzed the behavioral videos. PLJ wrote the main manuscript text. PLJ and KRM analyzed data and prepared Figs. 2, 3, 4. CD prepared Fig. 1 and supplementary figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jones, P.L., Martin, K.R., Prachand, S.V. et al. Compound-Specific Behavioral and Enzymatic Resistance to Toxic Milkweed Cardenolides in a Generalist Bumblebee Pollinator. J Chem Ecol 49, 418–427 (2023). https://doi.org/10.1007/s10886-023-01408-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-023-01408-3