Abstract
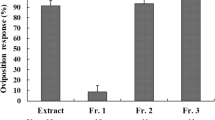
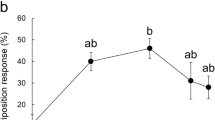
The common grass yellow butterfly, Eurema mandarina is a Fabaceae-feeding species, the females of which readily oviposit on Albizia julibrissin and Lespedeza cuneata in mainland Japan. We previously demonstrated that the methanolic leaf extracts of these plants, and their highly polar aqueous fractions strongly elicit female oviposition. Furthermore, the three subfractions obtained by ion-exchange chromatographic separation of the aqueous fraction have been found to be less effective alone, but synergistically stimulate female oviposition when combined. This indicates that female butterflies respond to multiple compounds with different acidity. We have previously identified d-pinitol from the neutral/amphoteric subfractions and glycine betaine from the basic subfractions as oviposition stimulants of E. mandarina. The present study aimed to identify active compounds in the remaining acidic subfractions of A. julibrissin and L. cuneata leaf extracts. GC-MS analyses of trimethylsilyl-derivatized samples revealed the presence of six compounds in the acidic subfractions. In bioassays using these authentic chemicals, erythronic acid (EA) and threonic acid (TA) were moderately active in eliciting oviposition responses in E. mandarina, with their d-isomers showing slightly higher activity than their l-isomers. Female responsiveness differed between d-EA and l-TA, the major isomers of these compounds in plants, with the response to d-EA reaching a plateau at concentrations above 0.005% and that to l-TA peaking at a concentration of 0.01%. The natural concentrations of d-EA and l-TA in fresh A. julibrissin and L. cuneata leaves were sufficient to stimulate oviposition. Furthermore, mixing 0.001% d-EA or 0.001% l-TA, to which females are mostly unresponsive, with 0.1% d-pinitol resulted in a synergistic enhancement of the oviposition response. These findings demonstrate that E. mandarina females utilize both polyhydroxy acids, EA and TA, as chemical cues for oviposition.



Similar content being viewed by others
Data Availability
All data used in the present genetic analyses are included in this published article and its supplementary information.
Change history
13 November 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10886-023-01463-w
References
Agrawal AA, Weber MG (2015) On the study of plant defence and herbivory using comparative approaches: how important are secondary plant compounds. Ecol Lett 18:985–991
Allio R, Nabholz B, Wanke S, Chomicki G, Pérez-Escobar OA, Cotton AM, Clamens A-L, Kergoat GJ, Sperling FAH, Condamine FL (2021) Genome-wide macroevolutionary signatures of key innovations in butterflies colonizing new host plants. Nat Comm 12:354
Awmack CS, Leather SR (2002) Host plant quality and fecundity in herbivorous insects. Annu Rev Entomol 47:817–844
Banno H (1984) The oviposition preference and larval food utilization in Eurema hecabe (Linnaeus) (Pieridae). Tyô to Ga 35:80–90
Berenbaum MR (1990) Evolution of specialization in insect-umbellifer associations. Annu Rev Entomol 35:319–343
Blake JD, Richards GN (1968) Problems of lactonization in the analysis of uronic acids. Carbohyd Res 8:275–281
Breeschoten T, van der Linden CFH, Ros VID, Schranz ME, Simon S (2022) Expanding the menu: are polyphagy and gene family expansions linked across Lepidoptera? Genome Biol Evol 14:evab283
Chew FS, Robbins RK (1984) Egg-laying in butterflies. In: Vane-Wright RI, Ackery PR (eds) The biology of butterflies. Academic Press, London, UK, pp 65–79
Chun MW, Schoonhoven LM (1973) Tarsal contact chemosensory hairs of the large white butterfly Pieris brassicae and their possible role in oviposition behaviour. Entomol Exp Appl 16:343–357
Cooper G, Rios AC (2016) Enantiomer excesses of rare and common sugar derivatives in carbonaceous meteorites. Proc Nat Am Soc 113:E3322–E3331
Ehrlich PR, Raven PH (1964) Butterflies and plants: a study in coevolution. Evolution 18:586–608
Fatouros NE, Lucas-Barbosa D, Weldegergis BT, Pashalidou FG, van Loon JJA, Dicke M, Harvey JA, Gols R, Huigens ME (2012) Plant volatiles induced by herbivore egg deposition affect insects of different trophic levels. PLoS ONE 7:e43607
Feeny P, Sachdev K, Rosenberry L, Carter M (1988) Luteolin 7-O-(6’’-O-malonyl)-β-d-glucoside and trans-chlorogenic acid: oviposition stimulants for the black swallowtail butterfly. Phytochemistry 27:3439–3448
Ferrer-Paris JR, Sámchez-Mercado A, Viloria ÁL, Donaldson J (2013) Congruence and diversity of butterfly-host plant associations at higher taxonomic levels. PLoS ONE 8:e63570
Glaubitz U, Erban A, Kopka J, Hincha DK, Zuther E (2015) High night temperature strongly impacts TCA cycle, amino acid and polyamine biosynthetic pathways in rice in a sensitivity-dependent manner. J Exp Bot 66:6385–6397
Gripenberg S, Mayhew PJ, Parnell M, Roslin T (2010) A meta-analysis of preference-performance relationships in phytophagous insects. Ecol Lett 13:383–393
Haribal M, Feeny P (2003) Combined roles of contact stimulant and deterrents in assessment of host-plant quality by ovipositing zebra swallowtail butterflies. J Chem Ecol 29:653–670
Haribal M, Renwick JAA (1996) Oviposition stimulants for the monarch butterfly: flavonol glycosides from Asclepias curassavica. Phytochemistry 41:139–144
Helsper JP, Loewus FA (1982) Metabolism of l-threonic acid in Rumex x acutus L. and Pelargonium crispum (L.) L’Hér. Plant Physiol 69:1365–1368
Honda K (1990) Identification of host-plant chemicals stimulating oviposition by swallowtail butterfly, Papilio protenor. J Chem Ecol 16:325–337
Honda K (1995) Chemical basis of differential oviposition by lepidopterous insects. Arch Insect Biochem Physiol 30:1–23
Honda K, Nishii W, Hayashi N (1997a) Oviposition stimulants for sulfur butterfly, Colias erate poliographys: cyanoglucosides as synergists involved in host preference. J Chem Ecol 23:323–331
Honda K, Hayashi N, Abe F, Yamauchi T (1997b) Pyrrolizidine alkaloids mediate host-plant recognition by ovipositing females of an old world danaid butterfly, Idea leuconoe. J Chem Ecol 23:1703–1713
Honda K, Ômura H, Hayashi N, Abe F, Yamauchi T (2001) Oviposition-stimulatory activity of phenanthroindolizidine alkaloids of host-plant origin to a danaid butterfly, Ideopsis similis. Physiol Entomol 26:6–10
Honda K, Ômura H, Hayashi N, Abe F, Yamauchi T (2004) Conduritols as oviposition stimulants for the danaid butterfly, Parantica sita, identified from a host plant, Marsdenia tomentosa. J Chem Ecol 30:2285–2296
Honda K, Minematsu H, Muta K, Ômura H, Nishii W (2012) d-Pinitol as a key oviposition stimulant for sulfur butterfly, Colias erate: chemical basis for female acceptance of host- and non-host plants. Chemoecology 22:55–63
Huang X, Renwick JAA (1994) Relative activities of glucosinolates as oviposition stimulants for Pieris rapae and P. napi oleracea. J Chem Ecol 20:1025–1037
Huang X, Renwick JAA, Sachdev-Gupta K (1994) Oviposition stimulants in Barbarea vulgaris for Pieris rapae and P. napi oleracea: isolation, identification and differential activity. J Chem Ecol 20:423–438
Itoh Y, Okumura Y, Fujii T, Ishikawa Y, Ômura H (2018) Effects of mating on host selection by female small white butterflies Pieris rapae. J Comp Physiol A 204:245–255
Jaenike J (1990) Host specialization in phytophagous insects. Annu Rev Ecol Syst 21:243–273
Janz N, Nylin S (1998) Butterflies and plants: a phylogenetic study. Evolution 52:486–502
Kato Y (2000) Host-plant adaptation in two sympatric types of the butterfly Eurema hecabe (L.) (Lepidoptera: Pieridae). Entomol Sci 3:459–463
Length R (2019) Emmeans: estimated marginal means, aka least-squares means. https://CRAN.R-project.org/package=emmeans
Lewinsohn TM, Novotny V, Basset Y (2005) Insects on plants: diversity of herbivore assemblages revisited. Annu Rev Ecol Evol Syst 36:597–620
Miura D, Tanaka H, Wariishi H (2004) Metabolomic differential display analysis of the white-rot basidiomycete Phanerochaete chrysosporium grown under air and 100% oxygen. FEMS Microbiol Lett 234:111–116
Mukae S, Ohashi T, Matsumoto Y, Ohta S, Ômura H (2016) d-Pinitol in Fabaceae: an oviposition stimulant for the common grass yellow butterfly, Eurema mandarina. J Chem Ecol 42:1122–1129
Murphy SM, Feeny P (2006) Chemical facilitation of a naturally occurring host shift by Papilio machaon butterflies (Papilionidae). Ecol Monog 76:399–414
Muto-Fujita A, Takemoto K, Kanaya S, Nakazato T, Tokimatsu T, Matsumoto N, Kono M, Chubachi Y, Ozaki K, Kotera M (2017) Data integration aids understanding of butterfly-host plant networks. Sci Rep 7:43368
Nakayama T, Honda K (2019) An oviposition stimulant for a Magnoliaceae-feeding swallowtail butterfly, Graphium doson, from its primary host plant, Michelia compressa. J Chem Ecol 45:926–933
Nakayama T, Honda K, Ômura H, Hayashi N (2003) Oviposition stimulants for the tropical swallowtail butterfly, Papilio polytes, feeding on a rutaceous plant, Toddalia asiatica. J Chem Ecol 29:1621–1634
Nishida R, Fukami H (1989) Oviposition stimulants of an Aristolochiaceae-feeding swallowtail butterfly, Atrophaneura alcinous. J Chem Ecol 15:2565–2575
Ohashi T, Ohta S, Ômura H (2019a) The role of N,N,N-trimethylglycine in oviposition of Eurema mandarina on Albizia julibrissin. J Chem Ecol 45:371–377
Ohashi T, Ohta S, Ômura H (2019b) A cyanogenic glucoside of Trifolium repens deters oviposition by the common grass yellow Eurema mandarina. Physiol Entomol 44:222–229
Ohsugi T, Nishida R, Fukami H (1991) Multi-component system of oviposition stimulants for a Rutaceae-feeding swallowtail butterfly, Papilio xuthus (Lepidoptera: Papilionidae). Appl Entomol Zool 26:29–40
Ômura H (2018) Plant secondary metabolites in host selection of butterfly. In: Tabata J (ed) Chemical ecology of insects: applications and associations with plants and microbes. CRC Press, Boca Raton, FL, pp 3–27
Ono H, Nishida R, Kuwahara Y (2000a) A dihydroxy-γ-lactone as an oviposition stimulant for the swallowtail butterfly, Papilio bianor, from the rutaceous plant, Orixa japonica. Biosci Biotechnol Biochem 64:1970–1973
Ono H, Nishida R, Kuwahara Y (2000b) Oviposition stimulant for a Rutaceae-feeding swallowtail butterfly, Papilio bianor (Lepidoptera: Papilionidae): hydroxycinnamic acid derivative from Orixa japonica. Appl Entomol Zool 35:119–123
Oyeyele SO, Zalucki MP (1990) Cardiac glycosides and oviposition by Danaus plexippus on Asclepias fruticosa in south-east Queensland (Australia), with notes on the effect of plant nitrogen content. Ecol Entomol 15:177–185
Papaj DR, Feeny P, Sachdev-Gupta K, Rosenberry L (1992) d-(+)-Pinitol, an oviposition stimulant for the pipevine swallowtail butterfly, Battus philenor. J Chem Ecol 18:799–815
R Development Core Team (2020) R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org
Renwick JAA (1989) Chemical ecology of oviposition in phytophagous insects. Experientia 45:223–228
Renwick JAA, Chew FS (1994) Oviposition behavior in Lepidoptera. Annu Rev Entomol 39:377–400
Richards LA, Dyer LA, Forister ML, Smilanich AM, Dodson CD, Leonard MD, Jeffrey CS (2015) Phytochemical diversity drives plant-insect community diversity. Proc Nat Sci USA 112:10973–10978
Sato K, Tanaka K, Touhara K (2011) Sugar-regulated cation channel formed by an insect gustatory receptor. Proc Natl Acad Sci USA 108:11680–11685
Savela M (2020) Lepidoptera and some other life forms. https://www.funet.fi/pub/sci/bio/life/insecta/lepidoptera/ditrysia/papilionoidea/pieridae/coliadinae/eurema/ Accessed 25 Apr 2022
Shirôzu T (2006) The standard of butterflies in Japan. Gakken, Tokyo. (in Japanese)
Thompson JN, Pellmyr O (1991) Evolution of oviposition behavior and host preference in Lepidoptera. Annu Rev Entomol 36:65–89
van Loon JJA, Blaakmeer A, Griepink FC, van Beek TA, Schoonhoven LM, de Groot A (1992) Leaf surface compound from Brassica oleracea (Cruciferae) induces oviposition by Pieris brassicae (Lepidoptera: Pieridae). Chemoecology 3:39–44
Wahlberg N (2001) The phylogenetics and biochemistry of host-plant specialization in Melitaeine butterflies (Lepidoptera: Nymphalidae). Evolution 55:522–537
Wahlberg N, Rota J, Braby MF, Pierce NE, Wheat CW (2014) Revised systematics and higher classification of pierid butterflies (Lepidoptera: Pieridae) based on molecular data. Zool Scr 43:641–650
Wheat CW, Vogel H, Wittstock U, Braby MF, Underwood D, Mitchell-Olds T (2007) The genetic basis of a plant-insect coevolutionary key innovation. Proc Nat Acad USA 104:20427–20431
Acknowledgements
We are thankful to Dr Tomoko Amimoto, at the Natural Science Center for Basic Research and Development (N-BARD), Hiroshima University, for the measurements of HR–ESI–MS.
Funding
This study was partially supported by the Grants-in-Aid for Scientific Research (KAKENHI) from the Japan Society for the Promotion of Science (JSPS) to HO (No. 19K06074).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and chemical analyses were performed by C.M., N.K., and H.Ô. Bioassays were performed by C.M., N.K., and Y.T. Statistical analyses were performed by H.Ô. The first draft of the manuscript was written by C.M. and N.K. and revised by H.Ô. with editorial inputs from T.F. and S.O. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
“In the originally published version of the article “Polyhydroxy acids as fabaceous plant components induce oviposition of the common grass yellow butterfly, Eurema mandarina” published in Journal of Chemical Ecology 49, 67–76 (2023), the manuscript contains an error in the concentration of d-pinitol, with some text and Figure 3 giving it as 0.01%, whereas the correct concentration is 0.1%. Figure 3 caption has been edited to ensure the correct display of information.” The original article has been corrected.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Matsunaga, C., Kanazawa, N., Takatsuka, Y. et al. Polyhydroxy Acids as Fabaceous Plant Components Induce Oviposition of the Common Grass Yellow Butterfly, Eurema Mandarina. J Chem Ecol 49, 67–76 (2023). https://doi.org/10.1007/s10886-022-01397-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-022-01397-9