Summary
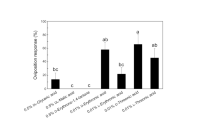
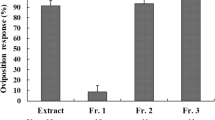
Chemicals present on the surface of cabbage (Brassica oleracea L.) leaves were extracted by dipping these leaves for 3 s in dichloromethane followed by a 3 s dip in methanol. When offered in dual choice bioassays using green paper cards as a substrate, the methanol extract stimulated oviposition activity byPieris brassicae L. (Lepidoptera: Pieridae) females. The oviposition stimulant was isolated using medium pressure liquid chromatography, reversed-phase HPLC, ion-pair HPLC and ion exchange chromatography. Using1H-NMR spectroscopy, the stimulant could be identified as glucobrassicin (3-indolyl-methyl-glucosinolate). When pure glucobrassicin was offered at a dose identical to that in the crude methanol extract, butterflies did not discriminate between these two substrates in a dual choice test. It is argued that a high sensitivity for indole glucosinolates as host recognition factors may confer an adaptive value for these specialist crucifer feeders. The nutritional significance of their precursor tryptophan and the non-volatile nature of the aglycones formed upon enzymic hydrolysis in damaged tissues are proposed as properties of indole glucosinolates that contribute to this possible adaptive advantage.
Similar content being viewed by others
References
Aplin RT, Ward RA, Rothschild M (1975) Examination of the large white and small white butterflies (Pieris spp.) for the presence of mustard oils and mustard oil glycosides. J Entomol (A) 50:73–78
Betz JM, Page SW (1990) Liquid chromatographic method for the determination of intact, non-derivatized glucosinolates from Brassicaceae. Pp 103–104in Proceedings of the Symposium Biology and Chemistry of Active Natural Substances. Bonn, July 1990
Boppré M (1983) Leaf scratching — a specialized behaviour of danaine butterflies (Lepidoptera) for gathering secondary plant substances. Oecologia 59:414–416
Chapman RF (1977) The role of the leaf surface in food selection by acridids and other insects. Colloques Internat C.N.R.S. 265:133–149
Chapman RF, Bernays EA (1989) Insect behavior at the leaf surface and learning as aspects of host plant selection. Experientia 45:215–222
Chew FS (1988a) Biological effects of glucosinolates. Pp 155–181in Cutler HG (ed.) Biologically Active Natural Products for Potential Use in Agriculture. ACS Symposium Series
Chew FS (1988b) Searching for defensive chemistry in the Cruciferae, or, do glucosinolates always control interactions of cruciferae with their potential herbivores and symbionts? No! Pp 81–112in Spencer KC (ed) Chemical Mediation of Coevolution. New York: Plenum Press
Dadd RH (1985) Nutrition: organisms. Pp 313–390in Kerkut GA, Gilbert LA (eds) Comprehensive Insect Physiology, Biochemistry and Pharmacology, Vol. 4. New York: Pergamon Press
David WAL, Gardiner BOC (1952) Laboratory breeding ofPieris brassicae L. andApanteles glomerata L. Proc R Entomol Soc Lond (A) 27:54–56
David WAL, Gardiner BOC (1962) Oviposition and the hatching of the eggs ofPieris brassicae L. in a laboratory culture. Bull ent Res. 53:91–109
Dethier VG (1982) Mechanisms of host plant recognition. Entomol exp appl 31:49–56
Eigenbrode SD, Stoner KA, Shelton AM, Kain WC (1991) Characteristics of glossy leaf waxes associated with resistance to diamond back moth (Lepidoptera: Plutellidae) inBrassica oleracea. J Econ Entomol 84:1609–1618
Feeny P, Rosenberry L, Carter M (1983) Chemical aspects of oviposition behaviour in butterflies. Pp 27–76in Ahmad S (ed) Herbivorous Insects: Host-Seeking Behavior and Mechanisms. New York: Academic Press
Feeny P, Sachdev K, Rosenberry L, Carter M (1988) Luteolin 7-O-(6′′-O-malonyl)-β-D-glucoside andtrans-chlorogenic acid: oviposition stimulants for the black swallowtail butterfly. Phytochemistry 27:3439–3448
Fenwick GR, Heany RK, Mullin WJ (1983) Glucosinolates and their breakdown products infood and food plants. CRC Critical Reviews in Food Science and Nutrition 18:123–201
Fraenkel GS (1959) The raison d'être of secondary plant substances. Science 129:1466–1470
Gödde J (1991) Perireceptor-events modulate spike responses in labellar largest taste hairs ofProtophormia. P 165in Elsner N, Penzlin H (eds) Synapse, Transmission, Modulation. Proc 19th Göttingen Neurobiol Conf. Suttgart: Georg Thieme Verlag
Heany RK, Fenwick GR (1980) Glucosinolates inBrassica vegetables. Analysis of 22 varieties of Brussels Sprout (Brassica oleracea var.gemmifera). J Sci Food Agric 31:785–793
Honda K (1986) Flavanone glycosides as oviposition stimulants in a papilionid butterfly,Papilio protenor. J Chem Ecol 12:1999–2010
Kaissling KE & Thorson (1980) Insect olfactory sensilla: structural, chemical and electrical aspects of the functional organization. Pp 261–282in Sattelle JB, Hall LM, Hildebrand JG (eds) Receptors for Neurotransmitters, Hormones and Pheromones in Insects. Amsterdam: Elsevier/North Holland
Jermy T (1984) Evolution of insect/host plant relationships. Am Nat 124:609–630
Kolb GK, Scherer C (1982) Experiments on wavelength-specific behavior ofPieris brassicae L. during drumming and egg-laying. J Comp Physiol 149:325–332
Laing JE, Levin DB (1982) A review of the biology and a bibliography ofApanteles glomeratus (L.) (Hymenoptera: Braconidae). Biocontrol News and Information 3:7–23
Loon JJA van, Eeuwijk FA van (1989) Chemoreception of amino acids in larvae of two species ofPieris. Physiol Entomol 14:459–469
Loon JJA van, Meer MMM van (1991) Chemosensory perception of leaf surface chemicals by ovipositingPieris brassicae L. butterflies. Proc Exp Appl Entomol 2:56–61
Ma WC, Schoonhoven LM (1973) Tarsal contact chemosensory hairs of the large white butterflyPieris brassicae and their possible role in oviposition behaviour. Entomol exp appl 16:343–357
McDanell R, McLean AEM, Hanley AB, Heany RK, Fenwick GR (1988) Chemical and biological properties of indole glucosinolates (glucobrassicin): a review. Food Chem Toxicol 26:59–70
Reed DW, Pivnick KA, Underhill EW (1989) Identification of chemical oviposition stimulants for the diamondback moth,Plutella xylostella, present in three species of Brassicaceae. Entomol exp appl 53:277–286
Renwick JAA, Radke CD (1983) Chemical recognition of host plants for oviposition by the cabbage butterfly,Pieris rapae (Lepidoptera: Pieridae). Environ Entomol 12:446–450
Renwick JAA, Radke CD (1988) Sensory cues in the host selection for oviposition by the cabbage butterfly,Pieris rapae. J Insect Physiol 34:251–257
Renwick JAA, Radke CD, Sachdev-Gupta K, Städler E (1992) Leaf surface chemicals stimulating oviposition byPieris rapae (Lepidoptera: Pieridae) on cabbage. Chemoecology 3:33–38
Rodman JE, Chew FS (1980) Phytochemical correlates of herbivory in a community of native and naturalized Cruciferae. Biochem Syst Ecol 8:43–50
Schoonhoven LM (1967) Chemoreception of mustard oil glucosides in larvae ofPieris brassicae. Proc Roy Acad Amsterdam Series C, 70:556–568
Schoonhoven LM (1969) Gustation and foodplant selection in some lepidopterous larvae. Entomol exp appl 12:555–564
Siegel S (1956) Nonparametric Statistics for the Behavioral Sciences. New York: John Wiley
Städler E (1986) Oviposition and feeding stimuli in leaf surface waxes. Pp 105–121in Juniper BE, Southwood TRE (eds) Insects and the Plant Surface. London: Edward Arnold
Städler E, Roessingh P (1991) Perception of surface chemicals by feeding and ovipositing insects. Pp 71–86in Szentesi A, Jermy T (eds) Proc 7th Int Symp Insect-Plant Relationships. Symp Biol Hung 39. Budapest: Akadémia Kiadó
Traynier RMM (1979) Long term changes in the oviposition behavior of the cabbage butterflyPieris rapae induced by contact with plants. Physiol Entomol 4:87–96
Traynier RMM (1984) Associative learning in the ovipositional behaviour of the cabbage butterfly,Pieris rapae. Physiol Entomol 9:465–472
Traynier RMM (1986) Visual learning in assays of sinigrin solution as an oviposition releaser for the cabbage butterfly,Pieris rapae. Entomol exp appl 40:25–33
Traynier RMM, Hines ER (1987) Probes by aphids indicated by stain induced fluorescence in leaves. Entomol exp appl 45:198–201
Traynier RMM, Truscott RJW (1991) Potent natural egg-laying stimulant for cabbage butterflyPieris rapae. J Chem Ecol 17:1371–1380
Van Etten CH, Tookey HL (1979) Chemistry and biological effects of glucosinolates. Pp 471–500in Rosenthal GA, Janzen DH (eds) Herbivores, Their Interaction with Secondary Plant Metabolites. New York: Academic Press
Verschaffelt E (1910) The cause determining the selection of food in some herbivorous insects. Proc Roy Acad Amsterdam 13:536–542
Vet LEM, Dicke M (1992) Ecology of infochemical use by natural enemies in a tritrophic context. Annu Rev Entomol 37:141–172
Whitman DC (1988) Plant natural products as parasitoid foraging cues. Pp 386–396in Cutler HG (ed.) Biologically Active Natural Products. Washington DC: American Chemical Society
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van Loon, J.J.A., Blaakmeer, A., Griepink, F.C. et al. Leaf surface compound fromBrassica oleracea (Cruciferae) induces oviposition byPieris brassicae (Lepidoptera: Pieridae). Chemoecology 3, 39–44 (1992). https://doi.org/10.1007/BF01261455
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01261455