Abstract
Anagrus atomus (L.) is an egg parasitoid involved in the biological control of Empoasca vitis (Göthe) in vineyards. Sex pheromones play a crucial role in mate finding for several parasitoid species and could be used for monitoring under field conditions. We carried out laboratory and field studies aimed at assessing the existence and identity of a possible A. atomus sex pheromone. We found that males were significantly attracted by virgin females independent of age. Males were not attracted to individuals of the same sex, but they were attracted by a crude extract from an unmated female and its polar fraction. Eugenol (4-allyl-2-methoxyphenol) was identified as the attractive substance and proved to be attractive not only in the olfactometer but also in another laboratory bioassay and under field conditions. Attraction of males, but not females, confirms that this is not an aggregation pheromone. This is the first sex-pheromone component identified in Mymaridae, however more compounds could be involved in the mating behaviour of A. atomus. The utility of a sex pheromone in A. atomus is discussed in the context of fitness returns.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mate finding is a crucial step in the mating system of insects and the use of reliable information may increase both the probability of finding a mate and mating success. Several stimuli (e.g. visual and tactile) can be used for this purpose but chemicals produced by members of both sexes are mainly involved (Symonds et al. 2009). Depending on the function, the range of activity of pheromones can vary. Highly volatile compounds released by females are used by males for long-range orientation during mate finding (> 2 cm), whereas chemicals of relative low volatility mediate male courtship behaviour at close range (Steiner et al. 2006). Sex pheromones play a crucial role in mate finding in most hymenopteran parasitoids (Kainoh 1999; Ruther 2013). Until now, the sex-pheromone components of more than 20 hymenopteran parasitoid species, belonging to ten families, have been identified (Eller et al. 1984; Hrabar et al. 2015; Kainoh 1999; Keeling et al. 2004; Nichols Jr et al. 2010; Pompanon et al. 1997; Quicke 1997; Ruther 2013; Ruther et al. 2011; Salerno et al. 2012). In the family Mymaridae, evidence of a sex pheromone has been reported for one species only (Cormier et al. 1998); however, no sex-pheromone components have been identified so far. Parasitoids’ sex pheromones are more often produced by females, however there is increasing evidence of sex pheromone production by males as well (Steiner et al. 2006; Steiner and Ruther 2009; Ruther 2013).
Interest in sex pheromones for use in integrated insect pest management is usually limited to pheromones released by herbivorous pest insects (Mainers and Peri 2013; Witzgall et al. 2010). Among the semiochemicals associated with natural enemies, mostly kairomones and synomones are considered in pest control strategies (James 2003, 2005; James and Price 2004; Rodriguez-Saona et al. 2012). However, the possibility of capturing natural enemies with traps baited with live females (Jewett and Carpenter 2001) or with lures containing their sex pheromones (De Lury et al. 1999; Eller et al. 1984; Gabrýs et al. 1997; Itadani and Ueno 2014; Suckling et al. 2002) has been exploited for research purposes.
Anagrus atomus L. (Hymenoptera: Mymaridae) is a cosmopolitan egg parasitoid of leafhoppers (Arnò et al. 1988; Chiappini 1987; Huber 1986; Matteucig and Viggiani 2008; Triapitsyn 1998; Waloff and Jervis 1987) and plays an important role in the biological control of the green leafhopper Empoasca vitis (Göthe) (Hemiptera: Cicadellidae) in European vineyards (Böll and Hermann 2004; Böll and Schwappach 2003; Boller et al. 2004; Cerutti et al. 1991; Hermann and Eichler 2000; Pavan and Picotti 1993, 2009; Picotti and Pavan 1993; van Helden and Decante 2001; Vidano et al. 1988; Zanolli and Pavan 2011, 2013). Anagrus atomus was suspected to be a complex of species, specialized on different leafhopper species, distinguishable on the basis of morphological characters, cuticular hydrocarbons and genetic analysis (Chiappini 1987; Chiappini et al. 1996; de León et al. 2009; Floreani et al. 2006; Nugnes et al. 2017; Triapitsyn 2015; Trjapitzin and Chiappini 1994; Viggiani and Nugnes 2018; Zanolli et al. 2016). However, an extensive recent review, considering both morphological characters and genetic analysis, suggested that the variability is intraspecific, rather than interspecific (Triapitsyn et al. 2020).
Anagrus atomus overwinters inside the eggs of alternative hosts on spontaneous vegetation surrounding vineyards (Boller et al. 2004; Ponti et al. 2003; Viggiani et al. 2006; Zanolli and Pavan 2011). In spring, the parasitoid moves into the vineyards where it completes several generations per year in E. vitis eggs (Cerutti et al. 1991). In autumn, A. atomus migrates back to the surrounding vegetation, where overwintering eggs are laid in eggs of leafhoppers other than E. vitis (Zanolli and Pavan 2011, 2013).
As with many other parasitoids, the mating system of A. atomus is characterized by protandry, with males emerging first followed by females (Zanolli and Pavan 2013). The sex ratio is generally 1:1 (Zanolli and Pavan 2011). Anagrus atomus females begin to lay eggs as soon as they emerge and have a life expectancy of 9 days at 24 °C (Agboka et al. 2004). In A. atomus, as in most hymenopteran parasitoids, male offspring emerge from unfertilized haploid eggs (arrhenotokous parthenogenesis) and female offspring from fertilized diploid eggs (Choudhury and Copland 2003; Heimpel and De Boer 2008).
In male parasitoids, the perception of the female sex pheromone is often followed by wing fanning (Böttinger et al. 2020; Vinson 1972). This behaviour has also been exploited for the purpose of pheromone isolation (Nazzi et al. 1995). When males of the closely related species A. incarnatosimilis Soyka and A. breviphragma Soyka recognize a virgin female, they raise their wings perpendicular to the dorsum and quickly move towards the female (Moratorio and Chiappini 1995). In A. breviphragma, mated females can still attract males such that, occasionally, a second insemination can occur (Moratorio and Chiappini 1995).
This study was carried out to ascertain the occurrence and identity of a female sex pheromone in the parasitoid A. atomus through behavioural bioassays and chemical analyses. The research included three consecutive steps: (1) verification of the existence of a female sex pheromone through behavioural bioassays; (2) identification of the chemical eliciting a response in males; (3) laboratory and field tests of the attractiveness of the identified compound towards males.
The information gathered on the female sex pheromone of A. atomus could be useful to monitor wasp populations in the field, to delineate their spatial distributions and to determine potential sources of parasitoids in the context of habitat management strategies.
Methods and Materials
Insect Rearing
Anagrus atomus was supplied by Biowise (Petworth, West Sussex, UK) as parasitized eggs of the leafhopper Hauptidia maroccana (Melichar) (Hemiptera: Cicadellidae) reared on Primula sp. plants. Parasitized eggs inside the leaf portions were individually isolated in vials (1.5 × 10 cm) and kept in a climatic chamber (Sanyo Versatile Environmental Test Chamber) at 60 ± 5% RH and 24 ± 1 °C with a daily light/dark cycle of 16:8 h. Honey was supplied to newly emerged adults used for bioassays. The vials were checked daily for adult emergence. Unmated males and females to be used in the bioassays were maintained in the same vial where development took place until use. Mated females were obtained by putting couples of newly emerged females and males in a vial until mating was observed and then placed back to their respective vial. After emergence parasitoid wasps were maintained under the same conditions as described above.
Olfactometer Bioassays
All behavioural experiments were performed using a four-arms olfactometer (diameter 10 cm) (Pettersson 1970; Vet et al. 1983). Stimuli to be tested (i.e. live insects, extracts and pure substances on a strip of filter paper) were placed in a syringe connected with an arm upwind of the exposure chamber and closed at the opposite end with a mesh, so that one field of the olfactometer was treated with the odour to be tested and three others arms served as odourless control fields. The olfactometer was placed in a dark cardboard box to exclude the interference of possible external visual cues. All bioassays were carried out between 10:00 am and 6:00 pm at 24 ± 1.5 °C, in a darkened room. The olfactometer was illuminated from the top under a 7500 lx light source. The air stream was maintained at 400 ml/min. One hour before the beginning of an experimental session, parasitoid wasps in vials were placed in the test room to get acclimatized to experimental conditions. To avoid any asymmetrical bias, the olfactometer was rotated 90° clockwise after each bioassay. Parasitoid wasps were introduced individually through a hole in the centre of the olfactometer’s ceiling. The test started as soon as the insect entered the olfactometer and lasted for 10 mins. Each wasp was tested only once. The first choice made by the insects and the time spent in each odour field were recorded with the computer program “OLFA 1.0” (Nazzi 1995). After each test, the olfactometer was dismantled, scrupulously washed with 70% ethanol and then rinsed with water. If not otherwise specified in the different experiments, 12 parasitoid wasps per treatment were tested.
During the olfactometer assays, the male behaviour (i.e. general behaviour, wing fanning and locomotory activity) was also recorded.
Behavioural Evidence of a Female Attractive Pheromone
To ascertain the existence of a female sex pheromone in A. atomus, four experiments were conducted. Experiments 1 and 2 were carried out to test the hypothesis that A. atomus females release a pheromone to attract males and whether male responsiveness is influenced by the age and mating status of the female. In Experiment 1 and 2, the response of males towards 0 to 4-day-old unmated females and towards 0 to 3-day-old mated females was evaluated, respectively. 0-day-old females were tested within 24 h from the emergence.
Experiment 3 was carried out to test the possible attraction of males to conspecific males, using 0 to 1-day-old unmated males both as bait and test insects.
Experiment 4 aimed at testing the response of males to the crude ether extract of 0 to 1-day-old unmated females. For this purpose, twelve 0 to 1-day-old unmated females were killed by freezing (30 mins, −20 °C) and then extracted with 120 μl of ether at room temperature for 15 mins. The resulting extracts were stored at −20 °C until use. For each of the 12 replicates of the bioassay, one female equivalent of the extract (10 μl) was applied to a strip of filter paper (absorbent paper 500 μm, 0.5 × 3.0 cm), and the solvent was allowed to evaporate. Then the strip was inserted into the syringe connected to the treated arm of the four-arm olfactometer. For each replicate a new strip of paper was used.
Identification of a Candidate Pheromone
To isolate and identify the female sex pheromone demonstrated as above, we carried out Experiment 5. This involved testing the biological activity of two fractions of the female extract using 0 to 1-day-old unmated males as test insects. Both males and females were collected and frozen within 24 h after emergence without feeding them. Ether extracts of A. atomus females and males were fractionated by liquid chromatography, and the resulting fractions bioassayed as usual. Batches of 15 freshly emerged females were extracted with 150 μl of ether at room temperature as described above, then the extract was loaded on a column packed with 100 mg silica gel (200–400 mesh, pore size 60 Å. Sigma Aldrich US) and eluted sequentially with 1 ml each of hexane and ether. One female equivalent of each fraction in 10 μl of the solvent was tested in the olfactometer against 16 individual A. atomus males. Ether and hexane alone were also tested.
Chemical Analysis of the Crude Extract
Ether extracts of males and females (N = 10 for each sex) were prepared as described above and analysed by coupled gas chromatography-mass spectrometry (GC-MS) on a Varian 3400 gas chromatograph coupled with a Varian Saturn 2000 mass spectrum detector, equipped with CIP-SIL 8 capillary column (30 m × 0.25 mm I.D.; 0.25 μm thickness). The oven temperature was 50 °C for 1 min, followed by a ramp to 320 °C at 10 °C/min; final temperature was held for 2 mins. The carrier gas was He maintained at a constant flow rate of 1 ml/min. Injected volume was 1 μl (splitless) and the injection temperature was 250 °C. The interface temperature was maintained at 250 °C. Mass Spectrometry (MS) detection was performed with electron impact (EI) mode at 70 eV by operating in the fil-mul delay in the 40–650 amu range. The identification of the volatile compounds emitted by females but not by males was performed by comparison of crude extracts from males and females. Unknown spectra were identified using the NIST Library (National Institute of Standards and Technologies, US) as a reference for spectra and retention indexes.
Coinjection of the Female Crude Extract and Synthetic Eugenol
An ether extract of females (N = 50) was prepared as described above, reduced under nitrogen to 5 μl, and 2 μl of this extract analysed by GC-MS. Then 1 μl of a solution of synthetic eugenol in ether (0.01 μg/μl, Sigma Aldrich US) was added to the remaining extract and 1 μl of this mixture analysed by GC-MS. This analysis also served for the purpose of roughly quantifying the amount of eugenol associated with the major peak found in the female extract but not in the male one.
Testing of the Candidate Sex Pheromone Component
In Experiment 6, the activity of synthetic eugenol on males and females was evaluated in olfactometer bioassays. Responses of A. atomus males (N = 16) to eugenol were assessed at different doses (0.1, 0.5, 1, 5, 10 and 100 ng). Ether was used as a control as the treatment stimulus was dissolved in that solvent.
The response to eugenol was also tested with 16 individual A. atomus unmated females (N = 16) in order to exclude the possibility that the substance is an aggregation rather than a sex pheromone. Ether was used as control as above.
Cage Bioassays
To confirm the attractiveness of eugenol in the laboratory, the response of 0 to 1-day-old unmated males was tested in a Plexiglas’s cage with ventilation holes covered with mesh (30 × 60 × 50 cm) and exposed to natural light. Fifty μl of an eugenol solution corresponding to 50 ng of pure compound were pipetted onto a strip of filter paper (absorbent paper, 0.5 × 3.0 cm) and, after evaporation of the solvent, the strip was fixed in the middle part of a yellow sticky trap (15 × 5 cm). Another trap baited with 50 μl of ether was used as a control. Traps were hanging from the top of the cage at a distance of 40 cm from each other and males were released inside once a day. The bioassay lasted 5 days. Every 24 h, a batch of five males was released and the traps were replaced. The number of males captured on traps was counted.
Field Experiments
This study was carried out in an insecticide unsprayed vineyard (cultivar Tocai Friulano) located in a hilly grape-growing area (locality Buttrio, 46° 01′ latitude N, 13° 21′ longitude E, 90 m a.s.l.). Yellow sticky traps (11.5 × 24 cm) smeared with Temoocid® (Kollant S.r.l., Vigonovo, Venice, Italy) were used for A. atomus monitoring (Picotti and Pavan 1993; Zanolli and Pavan 2011). Six traps baited with eugenol were compared with six control traps. Lures were prepared with 9 μg of synthetic eugenol in glass micro-vials (1 × 3 cm) with perforated plastic caps. Under laboratory conditions vials used as bait released about 20 ng of eugenol per day, but under field conditions greater release is likely. On each baited trap a vial with eugenol was hanged in the upper part, while in the control traps empty vials were applied. Traps were distributed in the vineyard following a randomized blocks scheme with six replicates (rows). The distance between the control and the treated traps was at least 20 m both along the rows and between the contiguous rows. Traps were replaced twice a week and baits were changed at each replacement. Anagrus atomus males captured at each sampling interval were counted in the laboratory under a dissection microscope. Within the A. atomus species, the intraspecific taxa A. atomus and A. parvus Soyka were also distinguished (Zanolli et al. 2016).
Statistical Analysis
Wasp residence time in the treated arm was compared to the average time spent in the control arms of the olfactometer using a paired one-tailed t-test. This test was adopted because: i) residence time in the olfactometer arms appeared to be normally distributed according to Kolmogorov and Smirnov method; ii) no significant differences among the control arms were detected using ANOVA; iii) since the olfactometer was rotated every minute, residence time in control arms could be considered as belonging to the same population of data; iv) a paired test appeared to be important to account for the high inter-individual variability of test insects, since different wasps were used in each replicate of each bioassay; v) the same statistical approach was adopted in similar studies (e.g. Drakulic et al. 2015; Fancelli et al. 2018; Mao-xin et al. 2008).
To compare the male response to females of different ages or physiological status (unmated/mated), the proportions of residence time in the treated arm with respect to control arms were calculated and before statistical analysis data were arcsine transformed. To compare the male response to females of different age, ANOVA and Tukey tests were performed, whereas to compare the male response to unmated/mated females an unpaired t-test was performed.
Number of males captured on yellow sticky traps in cage bioassays was compared with a Binomial test.
To compare the total field captures of A. atomus males on the eugenol baited and control traps along the monitoring periods, the Wilcoxon matched-pairs signed-ranks test was applied considering sampling dates as replicates.
Statistical analyses were performed with GraphPad Instat 3.0 for Macintosh.
Results
Behavioural Evidence of a Female Produced Sex Pheromone
In Experiment 1 males were significantly attracted towards the olfactometer arm containing the odour of a virgin female (paired t test, N = 12; 0 days: t = 6.21, P < 0.001; 1 day: t = 7.12, P < 0.001; 2 days: t = 4.0, P = 0.002; 3 days: t = 3.86, P = 0.003; 4 days: t = 6.85, P < 0.001; Fig. 1). Attraction was not significantly affected by female age (unpaired ANOVA, df = 4, F = 0.87, P = 0.49).
Time spent by Anagrus atomus males (± standard deviation) in the arms of an olfactometer treated or not with the odour of one unmated A. atomus female of different ages or one-day-old A. atmous males. Asterisks mark statistically significant differences (** = P < 0.01; *** = P < 0.001; NS = no significant differences)
In Experiment 2, mated females also elicited a positive male response (Fig. 2). No significant attraction was found on day 2 after female mating, but significance resumed at day 3 (paired t test, N = 12; 0 days: t = 2.13, P = 0.028; 1 day: t = 2.60, P = 0.012; 2 days: t = 0.53, P = 0.30; 3 days: t = 2.70, P = 0.002). Overall, female age did not seem to affect male response (unpaired ANOVA, df = 3, F = 1.69, P = 0.18).
Moreover, in both Experiments 1 and 2, males exposed to the odour released by a virgin female showed wing fanning and increased locomotory activity. In particular, most males fan their wings in the presence of a virgin female, whereas only a small proportion of those exposed to the odour of a mated female did so.
The comparison between the proportion of time that males spent in the arm of the olfactometer treated with the odour of a virgin or a mated female revealed that 1- and 2-day-old males were significantly more attracted to unmated females than to mated ones (unpaired t test, N = 12; 0 days: t = 2.04, P = 0.054, 1 day: t = 3.10, P = 0.005; 2 days: t = 3.04, P = 0.006; 3 days: t = 0.47, P = 0.64; Fig. 3).
In Experiment 3, males did not stay longer in the arm of the olfactometer treated with the odour of a young male (paired t test, N = 12, t = 1.49, P = 0.08; Fig. 1).
In Experiment 4, males showed a strong attraction towards a one-female-equivalent of the crude extract of unmated females (paired t test, N = 16, t = 7.43, P < 0.001; Fig. 4). The male response to the extract was similar to that displayed to a living unmated female, although no wing fanning was noted in this case.
Time spent by Anagrus atomus males (± standard deviation) in the treated and control arms of an olfactometer. The stimuli used for treating one arm of the olfactometer are the whole extract of A. atomus unmated females, the apolar and polar fractions of A. atomus unmated females extracts (F1 hexane and F2 ether, respectively) and the two solvents alone (hexane and ether). Asterisks mark statistically significant differences (*** = P < 0.001; NS = no significant differences)
Identification of a Candidate Pheromone
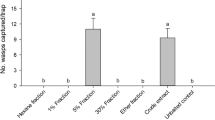
In Experiment 5, the more polar ether fraction of a female extract elicited a significant behavioural response from males (paired t test, N = 12, t = 5.05, P < 0.001), whereas neither the apolar fraction (paired t test, N = 12, t = 0.66, P = 0.26) nor ether (paired t test, N = 7, t = 1.43, P = 0.09) or hexane (paired t test, N = 12, t = 0.34, P = 0.37), used to extract the polar and the apolar hexane fractions respectively, significantly affected the male behaviour (Fig. 4).
Chemical Analysis of A. atomus Crude Whole Extract
The comparison of GC-MS analyses of virgin males’ and females’ crude extracts revealed only minor differences in the quantity of a few identified hydrocarbons. The most notable difference was one peak that was found only in the female extracts (Fig. 5). The peak was identified as eugenol by comparison of the mass-spectrum and retention time with of an authentic standard and confirmed by coinjection. The calculated amount of eugenol per female was less than 1 ng.
Testing of the Candidate Pheromone
In Experiment 6 male wasps were attracted to synthetic eugenol in the olfactometer responding to several doses ranging from 0.5 ng to 10 ng (paired t test, N = 16, 0.1 ng: t = 1.07, P = 0.15; 0.5 ng: t = 2.71, P = 0.008; 1 ng: t = 1.98, P = 0.033; 5 ng: t = 1.46, P = 0.08; 10 ng: t = 1.92, P = 0.037; 100 ng: t = 1.05, P = 0.16; Fig. 6). Unmated females did not show attraction towards the dose that appeared to be most attractive to males (paired t test, N = 16, t = 0.10, P = 0.46; Fig. 6).
Cage Bioassays
Under lab conditions yellow sticky traps baited with eugenol captured more caged males than control traps (binomial test, P = 0.02; Fig. 7).
Field Experiments
Under open field conditions yellow sticky traps baited with eugenol captured more A. atomus males than control traps (Wilcoxon matched-pairs signed-ranks test, W = 16.5, N = 13, P < 0.05; Fig. 8). If only the intraspecific taxa A. atomus was considered within A. atomus species, the differences were also significant (Wilcoxon matched-pairs signed-ranks test, W = 18.0, N = 13, P < 0.05).
Discussion
The attraction of A. atomus male wasps to both live virgin females and their crude extracts supports the hypothesis that a volatile sex pheromone is released by females, similar to what has previously been found in other hymenopteran parasitoids (De Lury et al. 1999; Eller et al. 1984; Nazzi et al. 1995; Ruther et al. 2000; Salerno et al. 2012; Simser and Coppel 1980). Attraction of males, but not females, confirms that this is not an aggregation pheromone.
Olfactometer and cage bioassays suggest that the attractant is a long-range pheromone, since the distance at which it is perceived by A. atomus males is greater than 2 cm (Keeling et al. 2004). The females of other Anagrus species, such as A. delicatus (Cronin and Strong 1990), A. incarnatosimilis and A. breviphragma (Moratorio and Chiappini 1995), mate at the emergence site. For this reason, it is likely they do not produce a long-range attractant analogous to other hymenopteran parasitoids showing the same reproductive behaviour (Godfray 1994). In fact, in this latter case, males emerge first and then wait for the female’s emergence to mate on site. In contrast, A. atomus does not mate at the emergence site because it parasitizes solitary eggs (Agboka et al. 2004), and, since only males are normally produced when females do not meet a male before egg laying, a female produced long range attractant facilitates male search. Natural selection may favour sex pheromone production if the population is not at sex ratio equilibrium or if there are advantages in having the capability of producing both sons and daughters (Godfray 1994). Metabolic costs are involved in the production of long-range sex pheromones and so it is likely that A. atomus females gain an advantage from mating.
Although some differences in male response to unmated and mated females were noted, they both appeared to be attractive to males. Therefore, the production/release of the pheromone by A. atomus females seems not to cease after mating, as would be expected if multiple matings occurred. Polyandry is known in other mymarids such as, for example Anaphes nitens (Santolamazza-Carbone and Pestaña 2010) and a second mating was occasionally observed also in A. breviphragma (Moratorio and Chiappini 1995). The fitness returns from multiple matings of old females are not clear since egg supply and quality usually decrease with mother age (Giron and Casas 2003). Nevertheless, a high tendency of old females to re-mate was observed in other wasps such as Nasonia vitripennis (Burton-Chellew et al. 2007) and could be related to the limited storage capacity of the spermatheca (Santolamazza-Carbone and Pestaña 2010). In haplodiploid hymenopteran species, sperm depletion followed by the oviposition of unfertilized male eggs is possible (Godfray 1990). In this case, since the evolutionary stable strategy sex ratio in panmictic populations is 1:1, as reported for A. atomus in previous studies (Zanolli and Pavan 2011), sperm-depleted females are likely to re-mate thus explaining the persistence of a female produced attractant after mating. The release of sex pheromone by mated females could be convenient if multiple matings increase lifespan of females, as observed in Trichogramma evanescens (Jacob and Boivin 2005), or if sex pheromones have multiple functions, as in Venturia canescens (Metzger et al. 2010).
The compound triggering male attraction was identified as eugenol (4-allyl-2-methoxyphenol), a sex pheromone component previously detected in male butterflies of the genus Amauris spp. (Lepidoptera, Danaidae) (Schulz et al. 1993) and in Bactrocera spp. (Symonds et al. 2009). No more compounds were identified in the active fraction of the crude extract, but the limited quantity of material extractable from females may have precluded the identification of other compounds. In fact, the magnitude of the biological effect elicited by several doses of the compound appeared to be smaller than that observed against live females. The presence of eugenol only in the female extract was confirmed also by analysing males and females of A. atomus emerged from grapevine leaves collected in the field (data not shown).
Furthermore, wing fanning has never been observed when extracts or pure compounds were tested in the bioassay. Since many hymenopteran parasitoid females produce sex pheromones that are constituted of a blend of two or more compounds, one attracting the males and others mediating the subsequent phases of the courtship behaviour (Quicke 1997; Ruther 2013), the hypothesis that more compounds involved in A. atomus reproduction are still to be identified is very likely. Nevertheless, this is the first study in which a sex-pheromone component was identified in Mymaridae.
Under open field conditions, eugenol significantly increased the already elevated captures of A. atomus males in yellow sticky traps, confirming the biological activity detected under laboratory conditions. Unbaited yellow sticky traps have already proved to be a valid tool to monitor field populations of A. atomus wasps (Picotti and Pavan 1993; Zanolli and Pavan 2011). A complete identification of the pheromone blend of this species could significantly increase the efficiency of such traps and their potential for monitoring of this beneficial insect as a bioindicator of the effects of chemical control and cultural practices on natural enemies (Gabrýs et al. 1997; Suckling et al. 2002).
References
Agboka K, Tounou AK, Phoeling H-M, Al-Moaalem R, Raupach K, Borgemeister C (2004) Life-table study of Anagrus atomus, an egg parasitoid of the green leafhopper Empoasca decipiens, at four different temperatures. Biocontrol 49:261–275. https://doi.org/10.1023/B:BICO.0000025385.52826.87
Arnò C, Alma A, Arzone A (1988) Anagrus atomus as egg parasite of Typhlocibinae (Rinchota Auchennorryncha). In: Arzone A, Vidano C (eds). Proc 6th Auchen Meeting, Turin, pp 611–615
Böll S, Hermann JV (2004) A long-term study on the population dynamics of the grape leafhopper (Empoasca vitis) and antagonistic mymarid species. J Pest Sci 77:33–42. https://doi.org/10.1007/s10340-003-0025-2
Böll S, Schwappach P (2003) Species spectrum, dominance relationships and populations dynamics of egg parasitoids (Mymaridae) of the grape leafhopper (Empoasca vitis Goethe) in the Franconian wine region. IOBC/WPRS Bull 26(8):173–180
Boller EF, Häni F, Poehling HM (2004) Ecological infrastructure. Ideabook on functional biodiversity at the farm level. Temperate zones of Europe, Verlag und Bezung, Lindau, Switzerland
Böttinger LC, Hüftlein F, Stökl J (2020) Mate attraction, chemical defense, and competition avoidance in the parasitoid wasp Leptopilina pacifica. Chemoecology 31:101–114. https://doi.org/10.1007/s00049-020-00331-3
Burton-Chellew MN, Sykes EM, Patterson S, Shuker DM, West SA (2007) The cost of mating and the relationship between body size and fitness in males of the parasitoid wasp Nasonia vitripennis. Evol Ecol Re 9:921–934. https://www.evolutionary-ecology.com/issues/v09n06/eear2197.pdf
Cerutti F, Baumgärtner J, Delucchi V (1991) The dynamics of grape leafhopper Empoasca vitis Göthe populations in southern Switzerland and the implications for habitat management. Biocontrol Sci Techn 1:177–194. https://doi.org/10.1080/09583159109355198
Chiappini E (1987) Ricerche sulla variabilità di Anagrus atomus (L.) (Hymenoptera Mymaridae) e di una specie affine presente su rovo. Boll Zool Agr Bachic Ser II (1986-1987) 19:71–97
Chiappini E, Triapitsyn SV, Donev A (1996) Key to Holarctic species of Anagrus Haliday (Hymenoptera: Mymaridae) with a review of the Nearctic and Palearctic (other than European) species and description of new taxa. J Nat Hist 30:551–595. https://doi.org/10.1080/00222939600770301
Choudhury DAM, Copland MJW (2003) A new record of thelytoky in the egg parasitoid Anagrus atomus (Linnaeus) (Hymenoptera: Mymaridae). PJBS 6:500–504. https://doi.org/10.3923/pjbs.2003.500.504
Cormier D, Royer L, Vigneault C, Panneton B, Boivin G (1998) Effect of female age on daily cycle of sexual pheromone emission in gregarious egg parasitoid Anaphes listronoti. J Chem Ecol 24:1595–1610. https://doi.org/10.1023/A:1020860326945
Cronin JT, Strong DR (1990) Biology of Anagrus delicates (Hymenoptera: Mymaridae), an egg parasitoid of Prokelisia marginata (Homoptera: Delphacidae). Ann Entomol Soc Am 83:846–854. https://doi.org/10.1093/aesa/83.4.846
de León JH, Triapitsyn SV, Matteucig G, Viggiani G (2009) Molecular and morphometric analyses of Anagrus erythroneurae Trjapitzin et Chiappini and Anagrus ustulatus Haliday (Hymenoptera: Mymaridae). Boll Lab Entomol Agr Filippo Silvestri, Portici 62:19–32
De Lury NC, Gries G, Gries R, Judd GJR, Brown JJ (1999) Sex pheromone of Ascogaster quadridentata, a parasitoid of Cydia pomonella. J Chem Ecol 25:2229–2245. https://doi.org/10.1023/A:1020813621977
Drakulic J, Caulfield J, Woodcock C, Jones SPT, Linforth R, Bruce TJA, Raya RV (2015) Sharing a host plant (wheat [Triticum aestivum]) increases the fitness of Fusarium graminearum and the severity of fusarium head blight but reduces the fitness of grain aphids (Sitobion avenae). Appl Environ Microbiol 81:3492–3501. https://doi.org/10.1128/AEM.00226-15
Eller FJ, Bartelt RJ, Jones RL, Kulman HM (1984) Ethyl (Z)-9-hexadecenoate a sex pheromone of Syndipnus rubiginosus, a sawfly parasitoid. J Chem Ecol 10:291–300. https://doi.org/10.1007/BF00987857
Fancelli M, Borges M, Laumann RA, Pickett JA, Birkett MA, Blassioli-Moraes MC (2018) Attractiveness of host plant volatile extracts to the asian citrus psyllid, Diaphorina citri, is reduced by terpenoids from the non-host cashew. J Chem Ecol 44:397–405. https://doi.org/10.1007/s10886-018-0937-1
Floreani C, Pavan F, Nazzi F (2006) Analysis of cuticular hydrocarbons in two Anagrus species (Hymenoptera: Mymaridae) as a tool to improve their correct identification. Can Entomol 138:348–356. https://doi.org/10.4039/n05-094
Gabrýs BJ, Gadomski HJ, Klukowski Z, Pickett JA, Sobota GT, Wadhams LJ, Woodcock CM (1997) Sex pheromone of cabbage aphid Brevicoryne brassicae: identification and field trapping of male aphids and parasitoids. J Chem Ecol 23:1881–1890. https://doi.org/10.1023/B:JOEC.0000006457.28372.48
Giron D, Casas J (2003) Mothers reduce egg provisioning with age. Ecol Lett 6:273–277. https://doi.org/10.1046/j.1461-0248.2003.00429.x
Godfray HCJ (1990) The causes and consequences of constrained sex allocation in haplodiploid animals. J Evol Biol 3:3–17. https://doi.org/10.1046/j.1420-9101.1990.3010003.x
Godfray HCJ (1994) Parasitoids: behavioural and evolutionary ecology. Princeton University Press, Princeton
Heimpel G, De Boer JG (2008) Sex determination in the Hymenoptera. Annu Rev Entomo 53:209–230. https://doi.org/10.1146/annurev.ento.53.103106.093441
Hermann JV, Eichler P (2000) Epidemiological studies of the grape leafhopper Empoasca vitis Goethe and its antagonistic egg parasitoids in the Franconian wine growing region (Germany). IOBC/WPRS Bull 23:115–121
Hrabar M, HuiMin Z, Gries R, Schaefer PW, Draper J, Britton R, Gries G (2015) (7E,11E)-3,5,9,11-tetramethyltridecadienal: sex pheromone of the strepsipteran Xenos peckii. J Chem Ecol 41:732–739. https://doi.org/10.1007/s10886-015-0613-7
Huber JT (1986) Systematics, biology, and hosts of the Mymaridae and Mymarommatidae (Insecta: Hymenoptera): 1758–1984. Entomography 4:185–243
Itadani H, Ueno T (2014) Chemically mediated mate finding of the polyphagous solitary parasitoid Itoplectis naranyae (Hymenoptera: Ichneumonidae). Ann Entomol Soc Am 107:288–294. https://doi.org/10.1603/AN12146
Jacob S, Boivin G (2005) Costs and benefits of polyandry in the egg parasitoid Trichogramma evanescens Westwood (Hymenoptera: Trichogrammatidae). Biocontrol 32:311–318. https://doi.org/10.1016/j.biocontrol.2004.10.009
James DG (2003) Synthetic herbivore-induced plant volatiles as field attractants for beneficial insects. Environ Entomol 32:977–982. https://doi.org/10.1603/0046-225X-32.5.977
James DG (2005) Further field evaluation of synthetic herbivore-induced plant volatiles as attractants for beneficial insects. J Chem Ecol 31:481–495. https://doi.org/10.1007/s10886-005-2020-y
James DG, Price TS (2004) Field-testing of methyl salicylate for recruitment and retention of beneficial insects in grapes and hops. J Chem Ecol 30:1613–1628. https://doi.org/10.1023/B:JOEC.0000042072.18151.6f
Jewett DK, Carpenter JE (2001) Seasonal abundance of a pupal parasitoid, Diapetimorpha introita (Hymenoptera: Ichneumonidae). Fla Entomol 84:50–54. http://hdl.handle.net/10113/27308
Kainoh Y (1999) Parasitoids. In: Hardie J, Minks K (eds) Pheromones of non-lepidopteran insects associated with agricultural plants. CABI Publishing, Wallingford, pp 383–404
Keeling CI, Plettner E, Slessor KN (2004) Hymenopteran semiochemicals. Top Curr Chem 239:133–177. https://doi.org/10.1007/b95452
Mainers Y, Peri E (2013) Chemical ecology of insect parasitoids: essential elements for developing effective biological control programmes. In: Wajnberg E, Colazza S (eds) Chemical ecology of insect parasitoids. Wiley-Blackwell, Chichester, pp 193–223
Mao-xin Z, Bing L, Shao-ying C, Guang-wen L, Xiong-fei P (2008) Repellent and oviposition deterrent activities of the essential oil from Mikania micrantha and its compounds on Plutella xylostella. Entomologia sinica 11:37–45. https://doi.org/10.1111/j.1744-7917.2004.tb00178.x
Matteucig G, Viggiani G (2008) Fenologia e ospiti di Anagrus gruppo atomus (Linnaeus) (Hymenoptera: Mymaridae) in Campania. Boll Lab Ent agr Filippo Silvestri 62:45–50
Metzger M, Fischbein D, Auguste A, Fauvergue X, Bernstein C, Desouhant E (2010) Synergy in information use for mate finding: demonstration in a parasitoid wasp. An Behav 79:1307–1315. https://doi.org/10.1016/j.anbehav.2010.03.003
Moratorio MS, Chiappini E (1995) Biology of Anagrus incarnatosimilis and Anagrus breviphragma (Hymenoptera: Mymaridae). Boll Zool Agr Bachic Serie II 27:143–162
Nazzi F (1995) OLFA 1.0: a computer program for collecting and analyzing behavioural data with the four-way olfactometer. Exeter Software. ISBN 0-925031-26-7
Nazzi F, Powell W, Wadhams L, Woodcock CM (1995) Sex pheromone of aphid parasitoid Praon volucre (Hymenoptera, Braconidae). J Chem Ecol 22:1169–1175. https://doi.org/10.1007/BF02027952
Nichols WJ Jr, Cossé AA, Bartelt RJ, King BH (2010) Methyl 6-methylsalicylate: a female-produced pheromone component of the parasitoid wasp Spalangia endius. J Chem Ecol 36:1140–1147. https://doi.org/10.1007/s10886-010-9855-6
Nugnes F, Bernardo U, Viggiani G (2017) An integrative approach to species discrimination in the Anagrus atomus group sensu stricto (Hymenoptera: Mymaridae), with a description of a new species. Syst Biodivers 15:582–599. https://doi.org/10.1080/14772000.2017.1299811
Pavan F, Picotti P (1993) Dinamica di popolazione di Empoasca vitis (Göthe) (Homoptera Cicaldellidae) e del suo parassitoide oofago Anagrus atomus (Linnaeus) (Hymenoptera, Mymaridae) in vigneti e actinidieti contigui. Mem Soc Entomol Ital 72:163–173
Pavan F, Picotti P (2009) Influence of grapevine cultivars on the leafhopper Empoasca vitis and its eggs parasitoids. Biocontrol 54:55–63. https://doi.org/10.1007/s10526-008-9151-3
Pettersson J (1970) An aphid sex attractant. I. Biological studies. Entomol Scand 1:63–73. https://doi.org/10.1163/187631270X00357
Picotti P, Pavan F (1993) Studi su Anagrus atomus (Linnaeus) (Hymenoptera, Mymaridae) parassitoide oofago di Empoasca vitis (Goethe) (Homoptera, Cicaldellidae) su vite. 1. Dinamica di popolazione in assenza di trattamenti insetticidi. Boll Lab Entomol Agr Filippo Silvestri 48:105–115
Pompanon F, De Schepper B, Mourer Y, Fouillet P, Bouletreau M (1997) Evidence for a substrate-borne sex pheromone in the parasitoid wasp Trichogramma brassicae. J Chem Ecol 23:1349–1360. https://doi.org/10.1023/B:JOEC.0000006468.19993.70
Ponti L, Ricci C, Torricelli R (2003) The ecological role of hedges on population dynamics of Anagrus spp. (Hymenoptera: Mymaridae) in vineyards of Central Italy. IOBC/WPRS Bull 26:117–122
Quicke DLJ (1997) Parasitic wasps. Chapman and Hall, London
Rodriguez-Saona C, Blaauw BR, Isaacs R (2012) Manipulation of natural enemies in agroecosystems: habitat and semiochemicals for sustainable insect pest control. In: Larramendy ML, Saloneski S (eds) Integrate pest management and pest control - current and future tactics. InTech Publisher, Germany, pp 89–126
Ruther J (2013) Novel insights into pheromone-mediated communication in parasitic hymenopterans. In: Wajnberg E, Colazza S (eds) Chemical ecology of insect parasitoids. Wiley-Blackwell, Chichester, pp 112–144
Ruther J, Döring M, Steiner S (2011) Cuticular hydrocarbons as contact sex pheromone in the parasitoid Dibrachys cavus. Entomol Exp Appl 140:59–68. https://doi.org/10.1111/j.1570-7458.2011.01129.x
Ruther J, Homann M, Steidle JLM (2000) Female-derived sex pheromone mediates courtship behaviour in the parasitoid Lariophagus distinguendus. Entomol Exp Appl 96:265–274. https://doi.org/10.1046/j.1570-7458.2000.00705.x
Salerno G, Frati F, Iacovone A, Conti E, Peri E, Colazza S (2012) A female-produced short-range sex pheromone in the egg parasitoid Trissolcus brochymenae. Invertebr Biol 131:144–153. https://doi.org/10.1111/j.1744-7410.2012.00258.x
Santolamazza-Carbone S, Pestaña M (2010) Polyandry increases male offspring production in the quasi-gregarious egg parasitoid Anaphes nitens. Ethol Ecol Evolu 22:51–61. https://doi.org/10.1080/03949370903515984
Schulz S, Boppre M, Vane-Wright RI (1993) Specific mixtures of secretions from male scent organs of African milkweed butterflies (Danainae). Phil Trans R Soc Lon B 342:161–181. https://www.jstor.org/stable/3030144
Simser DH, Coppel HC (1980) Female-produced sex pheromone in Brachymeria lasus and B. intermedia (Hym.: Chalcididae). Entomophaga 25:373–380. https://doi.org/10.1007/BF02374700
Steiner S, Hermann N, Ruther J (2006) Characterization of a female-produced courtship pheromone in the parasitoid Nasonia vitripennis. J Chem Ecol 32:1687–1702. https://doi.org/10.1007/s10886-006-9102-3
Steiner S, Ruther J (2009) Mechanism and behavioural context of male sex pheromone release in Nasonia vitripennis. J Chem Ecol 35:416–421. https://doi.org/10.1007/s10886-009-9624-6
Suckling DM, Gibb AR, Burnip GM, Delury NC (2002) Can parasitoid sex pheromones help in insect biocontrol? A case study of codling moth (Lepidoptera: Tortricidae) and its parasitoid Ascogaster quadridentata (Hymenoptera: Braconidae). Environ Entomol 31:947–952. https://doi.org/10.1603/0046-225X-31.6.947
Symonds MRE, Moussalli A, Elgar M (2009) The evolution of sex pheromones in an ecologically diverse genus of flies. Biol J Linn Soc 97:594–603. https://doi.org/10.1111/j.1095-8312.2009.01245.x
Triapitsyn SV (1998) Anagrus (Hymenoptera: Mymaridae) egg parasitoids of Erythroneura spp. and other leafhoppers (Homoptera: Cicaldellidae) in north American vineyards and orchards: a taxonomic review. T Am Entomol Soc 124:77–112. https://www.jstor.org/stable/25078658
Triapitsyn SV (2015) Taxonomy of the genus Anagrus Haliday (Hymenoptera: Mymaridae) of the world: an annotated key to the described species, discussion of the remaining problems, and a checklist. Acta Zool Lilloana 59:3–50. http://www.lillo.org.ar/.../01.pdf
Triapitsyn SV, Rugman-Jones PF, Tretiakov PS, Daane KM, Wilson H (2020) Reassessment of molecular and morphological variation within the Anagrus atomus species complex (Hymenoptera: Mymaridae): egg parasitoids of leafhoppers (Hemiptera: Cicadellidae) in Europe and North America. J Nat Hist 54(27–28):1735–1758. https://doi.org/10.1080/00222933.2020.1827073
Trjapitzin SV, Chiappini E (1994) A new Anagrus (Hymenoptera: Mymaridae), an egg parasitoid of Erythroneura spp (Homoptera: Cicadellidae). Entomol News 105:137–140
van Helden M, Decante D (2001) The possibilities for conservation biocontrol as a management strategy Empoasca vitis. IOBC/WPRS Bull 24(7):291–297
Vet LEM, van Lenteren JC, Heymans M, Meelis E (1983) An airflow olfactometer for measuring olfactory responses of hymenopterous parasitoids and other small insects. Physiol Entomol 8:97–106. https://doi.org/10.1111/j.1365-3032.1983.tb00338.x
Vidano C, Arnò C, Alma A (1988) On the Empoasca vitis intervention threshold on vine (Rynchota, Auchennorryncha). In: Vidano C, Arzone A (eds) . Proceedings 6th Auchenorrhyncha meeting, Turin, pp 525–537
Viggiani G, Di Luca A, Matteucig G (2006) The egg parasitoids of the genus Anagrus (Hymenoptera: Mymaridae) as functional biodiversity of the vineyard agroecosystem. IOBC/WPRS Bull 29:157–160
Viggiani G, Nugnes F (2018) On a new species of Anagrus (Hymenoptera: Mymaridae) a note on the availability of the name Anagrus nepetellae Viggiani & Nugnes. J Entomol Acarol Res 50:7428. https://doi.org/10.4081/jear.2018.7428
Vinson SB (1972) Courtship behaviour and evidence for a sex pheromone in the parasitoid Campoletis sonorensis (Hymenoptera: Ichneumonidae). Environ Entomol 1:409–414. https://doi.org/10.1093/ee/1.4.409
Waloff N, Jervis MA (1987) Communities of parasitoids associated with leafhoppers and planthoppers in Europe. Adv Ecol Res 17:281–402. https://doi.org/10.1016/S0065-2504(08)60248-2
Witzgall P, Kirsch P, Cork A (2010) Sex pheromones and their impact on pest management. J Chem Ecol 36:80–100. https://doi.org/10.1007/s10886-009-9737-y
Zanolli P, Martini M, Mazzon L, Pavan F (2016) Morphological e molecular identification of Anagrus ‘atomus’ group (Hymenoptera: Mymaridae) individuals from different geographic areas and plant hosts in Europe. J Insect Sci 16:1–14. https://doi.org/10.1093/jisesa/iew017
Zanolli P, Pavan F (2011) Autumnal emergence of Anagrus wasps, egg parasitoids of Empoasca vitis, from grapevine leaves and their migration towards brambles. Agr Forest Entomol 13:423–433. https://doi.org/10.1111/j.1461-9563.2011.00546.x
Zanolli P, Pavan F (2013) Occurrence of different development time patterns induced by photoperiod in Anagrus atomus (Hymenoptera: Mymaridae), an egg parasitoid of Empoasca vitis (Homoptera: Cicadellidae). Physiol Entomol 38:269–278. https://doi.org/10.1111/phen.12029
Funding
Open access funding provided by Università degli Studi di Udine within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zanolli, P., Annoscia, D., Zanni, V. et al. Behavioural Evidence and Chemical Identification of a Female Sex Pheromone in Anagrus atomus (Hymenoptera: Mymaridae). J Chem Ecol 47, 534–543 (2021). https://doi.org/10.1007/s10886-021-01272-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-021-01272-z