Abstract
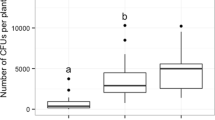
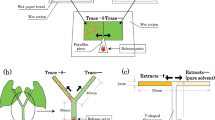
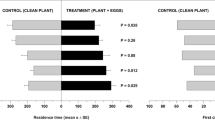
Mutualistic associations such as the fungal farms of insects are prone to parasitism and are consequently vulnerable to attack by weeds and pests. Therefore, efficient farm management requires quick detection of weeds for their elimination. Furthermore, if the available weedicides are non-specific, then the ability of insects to discriminate between crop and weeds becomes essential for targeted application of such compounds. Here, we demonstrate for the first time in fungus-farming insects, that worker castes of the fungus-growing termite Odontotermes obesus discriminate between their crop (Termitomyces) and the weedy (Pseudoxylaria) fungi, even if exposed to only fungal scents. Termites respond to the presence of fungal mycelium or scent alone, by burying the weed with the offered material such as soil or agar, possibly anointing the weed with chemicals in the process. The scent profiles of crop and weedy fungi are distinct and the differences are likely exploited by termites to selectively mount their defences. Sesquiterpene compounds such as aristolene and viridiflorol, which are absent from crop odours, may constitute the “weedy scent”. Our results provide a general mechanism of how other fungus-farming insects could avoid indiscriminate application of non-specific fungicides which could lead to poisoning their crops, and have bearing on the stability of the mutualism between termites and their crop fungus in the face of parasitism by weedy fungi.




Similar content being viewed by others
References
Aanen DK, de Fine Licht HH, Debets AJM et al (2009) High symbiont relatedness stabilizes mutualistic cooperation in fungus-growing termites. Science 326:1103–1106. https://doi.org/10.1126/science.1173462
Althoff DM (2014) Shift in egg-laying strategy to avoid plant defense leads to reproductive isolation in mutualistic and cheating yucca moths. Evolution 68:301–307. https://doi.org/10.1111/evo.12279
Baer B, Schmid-Hempel P (1999) Experimental variation in polyandry affects parasite loads and fitness in a bumble-bee. Nature 397:151–154. https://doi.org/10.1038/16451
Batra LR, Batra SWT (1966) Fungus-growing termites of tropical India and associated fungi. J Kans Entomol Soc 39:725–738
Batra LR, Batra SWT (1979) Termite-fungus mutualism. In: Batra LR (ed) Insect-fungus symbiosis: nutrition, mutualism, commensalism, Allanheld. Osmun & Co., Montclair, pp 117–163
Beemelmanns C, Ramadhar TR, Kim KH et al (2017) Macrotermycins A–D, glycosylated macrolactams from a termite-associated Amycolatopsis sp. M39. Org Lett 19:1000–1003. https://doi.org/10.1021/acs.orglett.6b03831
Biedermann PHW, Kaltenpoth M (2014) New synthesis: The chemistry of partner choice in insect-microbe mutualisms. J Chem Ecol 40:99–99. https://doi.org/10.1007/s10886-014-0382-8
Boomsma JJ, Aanen DK (2009) Rethinking crop-disease management in fungus-growing ants. Proc Natl Acad Sci U S A 106:17611–17612. https://doi.org/10.1073/pnas.0910004106
Borges RM (2015a) How to be a fig wasp parasite on the fig–fig wasp mutualism. Curr Opin Insect Sci 8:34–40. https://doi.org/10.1016/j.cois.2015.01.011
Borges RM (2015b) How mutualisms between plants and insects are stabilized. Curr Sci 108:1862–1868
Bot ANM, Rehner SA, Boomsma JJ (2001) Partial incompatibility between ants and symbiotic fungi in two sympatric species of Acromyrmex leaf-cutting ants. Evolution 55:1980–1991. https://doi.org/10.1554/0014-3820(2001)055[1980:PIBAAS]2.0.CO;2
Chouvenc T, Su N-Y (2012) When subterranean termites challenge the rules of fungal epizootics. PLoS One 7:e34484. https://doi.org/10.1371/journal.pone.0034484
Chouvenc T, Su N-Y, Elliott ML (2008) Interaction between the subterranean termite Reticulitermes flavipes (Isoptera: Rhinotermitidae) and the entomopathogenic fungus Metarhizium anisopliae in foraging arenas. J Econ Entomol 101:885–893
Chouvenc T, Robert A, Sémon E, Bordereau C (2012) Burial behaviour by dealates of the termite Pseudacanthotermes spiniger (Termitidae, Macrotermitinae) induced by chemical signals from termite corpses. Insect Soc 59:119–125. https://doi.org/10.1007/s00040-011-0197-3
Currie CR, Stuart AE (2001) Weeding and grooming of pathogens in agriculture by ants. Proc R Soc Lond B Biol Sci 268:1033–1039. https://doi.org/10.1098/rspb.2001.1605
Duringer P, Schuster M, Genise JF et al (2007) New termite trace fossils: Galleries, nests and fungus combs from the Chad basin of Africa (Upper Miocene–Lower Pliocene). Palaeogeogr Palaeoclimatol Palaeoecol 251:323–353. https://doi.org/10.1016/j.palaeo.2007.03.029
Fäldt J, Jonsell M, Nordlander G, Borg-Karlson A-K (1999) Volatiles of bracket fungi Fomitopsis pinicola and Fomes fomentarius and their functions as insect attractants. J Chem Ecol 25:567–590. https://doi.org/10.1023/A:1020958005023
Frederickson ME (2013) Rethinking mutualism stability: cheaters and the evolution of sanctions. Q Rev Biol 88:269–295. https://doi.org/10.1086/673757
Gerstner AT, Poulsen M, Currie CR (2011) Recruitment of minor workers for defense against a specialized parasite of Atta leaf-cutting ant fungus gardens. Ethol Ecol Evol 23:61–75. https://doi.org/10.1080/03949370.2010.529828
Hulcr J, Mann R, Stelinski LL (2011) The scent of a partner: ambrosia beetles are attracted to volatiles from their fungal symbionts. J Chem Ecol 37:1374–1377. https://doi.org/10.1007/s10886-011-0046-x
Hussain A, Tian M-Y, He Y-R et al (2010) Behavioral and electrophysiological responses of Coptotermes formosanus Shiraki towards entomopathogenic fungal volatiles. Biol Control 55:166–173. https://doi.org/10.1016/j.biocontrol.2010.08.009
Katariya L (2017) Ecology of fungus-farming by termites: Fungal populaton genetics and defensive mechanisms of termites against the parasitic fungus Pseudoxylaria. Ph D thesis. Indian Institute of Science, Bangalore
Katariya L, Ramesh PB, Gopalappa T, Borges RM (2017) Sex and diversity: The mutualistic and parasitic fungi of a fungus-growing termite differ in genetic diversity and reproductive strategy. Fungal Ecol 26:20–27. https://doi.org/10.1016/j.funeco.2016.11.003
Kim KH, Ramadhar TR, Beemelmanns C et al (2014) Natalamycin A, an ansamycin from a termite-associated Streptomyces sp. Chem Sci 5:4333–4338. https://doi.org/10.1039/C4SC01136H
Lamberty M, Zachary D, Lanot R et al (2001) Insect immunity: constitutive expression of a cysteine-rich antifungal and a linear antibacterial peptide in a termite insect. J Biol Chem 276:4085–4092. https://doi.org/10.1074/jbc.M002998200
Mburu DM, Ochola L, Maniania NK et al (2009) Relationship between virulence and repellency of entomopathogenic isolates of Metarhizium anisopliae and Beauveria bassiana to the termite Macrotermes michaelseni. J Insect Physiol 55:774–780. https://doi.org/10.1016/j.jinsphys.2009.04.015
Mburu DM, Ndung’u MW, Maniania NK, Hassanali A (2011) Comparison of volatile blends and gene sequences of two isolates of Metarhizium anisopliae of different virulence and repellency toward the termite Macrotermes michaelseni. J Exp Biol 214:956–962. https://doi.org/10.1242/jeb.050419
Mburu DM, Maniania NK, Hassanali A (2013) Comparison of volatile blends and nucleotide sequences of two Beauveria bassiana isolates of different virulence and repellency towards the termite Macrotermes michealseni. J Chem Ecol 39:101–108. https://doi.org/10.1007/s10886-012-0207-6
Milner R, Staples J, Lutton G (1998) The selection of an isolate of the hyphomycete fungus, Metarhizium anisopliae, for control of termites in Australia. Biol Control 11:240–247. https://doi.org/10.1006/bcon.1997.0574
Morelos-Juárez C, Walker TN, Lopes JFS, Hughes WOH (2010) Ant farmers practice proactive personal hygiene to protect their fungus crop. Curr Biol 20:R553–R554. https://doi.org/10.1016/j.cub.2010.04.047
Mueller UG, Gerardo NM, Aanen DK et al (2005) The evolution of agriculture in insects. Annu Rev Ecol Evol Syst 36:563–595. https://doi.org/10.1146/annurev.ecolsys.36.102003.152626
Myles TG (2002) Alarm, aggregation and defense by Reticulitermes flavipes in response to a naturally occurring isolate of Metarhizium anisopliae. Sociobiology 40:243–256
Nobre T, Koné NA, Konaté S et al (2011) Dating the fungus-growing termites’ mutualism shows a mixture between ancient codiversification and recent symbiont dispersal across divergent hosts. Mol Ecol 20:2619–2627. https://doi.org/10.1111/j.1365-294X.2011.05090.x
Poulsen M, Currie CR (2010) Symbiont interactions in a tripartite mutualism: Exploring the presence and impact of antagonism between two fungus-growing ant mutualists. PLoS One 5:e8748. https://doi.org/10.1371/journal.pone.0008748
R Core Team (2016) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna Available at https://www.R-project.org
Ranganathan Y, Borges RM (2010) Reducing the babel in plant volatile communication: using the forest to see the trees. Plant Biol 12:735–742. https://doi.org/10.1111/j.1438-8677.2009.00278.x
Roberts EM, Todd CN, Aanen DK et al (2016) Oligocene termite nests with in situ fungus gardens from the Rukwa Rift Basin, Tanzania, support a Paleogene African origin for insect agriculture. PLoS One 11:e0156847. https://doi.org/10.1371/journal.pone.0156847
Roisin Y, Everaerts C, Pasteels JM, Bonnard O (1990) Caste-dependent reactions to soldier defensive secretion and chiral alarm/recruitment pheromone in Nasutitermes princeps. J Chem Ecol 16:2865–2875. https://doi.org/10.1007/BF00979479
Rosengaus RB, Guldin MR, Traniello JF (1998) Inhibitory effect of termite fecal pellets on fungal spore germination. J Chem Ecol 24:1697–1706
Rosengaus RB, Jordan C, Lefebvre ML, Traniello JFA (1999) Pathogen alarm behavior in a termite: A new form of communication in social insects. Naturwissenschaften 86:544–548. https://doi.org/10.1007/s001140050672
RStudio Team (2016) RStudio: Integrated Development for R. RStudio, Inc., Boston Available at http://www.rstudio.com
Sands WA (1969) The association of termites and fungi. In: K. Krishna, & F. M. Weesner, (Eds.), Biology of Termites. Elsevier, pp 495–524
Schiestl FP, Steinebrunner F, Schulz C et al (2006) Evolution of ‘pollinator’- attracting signals in fungi. Biol Lett 2:401–404. https://doi.org/10.1098/rsbl.2006.0479
Schmid-Hempel P, Crozier RH (1999) Ployandry versus polygyny versus parasites. Philos Trans R Soc B Biol Sci 354:507–515. https://doi.org/10.1098/rstb.1999.0401
Sen R, Ishak HD, Estrada D et al (2009) Generalized antifungal activity and 454-screening of Pseudonocardia and Amycolatopsis bacteria in nests of fungus-growing ants. Proc Natl Acad Sci U S A 106:17805–17810. https://doi.org/10.1073/pnas.0904827106
Sherman PW, Seeley TD, Reeve HK (1988) Parasites, pathogens, and polyandry in social hymenoptera. Am Nat 131:602–610. https://doi.org/10.1086/284809
Spiteller P (2015) Chemical ecology of fungi. Nat Prod Rep 32:971–993. https://doi.org/10.1039/C4NP00166D
Thomas RJ (1987) Factors affecting the distribution and activity of fungi in the nests of macrotermitinae (Isoptera). Soil Biol Biochem 19:343–349
Um S, Fraimout A, Sapountzis P et al (2013) The fungus-growing termite Macrotermes natalensis harbors bacillaene-producing Bacillus sp. that inhibit potentially antagonistic fungi. Sci Rep. https://doi.org/10.1038/srep03250
Viana AMM, Frézard A, Malosse C et al (2001) Colonial recognition of fungus in the fungus-growing ant Acromyrmex subterraneus subterraneus (Hymenoptera: Formicidae). Chemoecology 11:29–36. https://doi.org/10.1007/PL00001829
Vining LC (1990) Functions of secondary metabolites. Annu Rev Microbiol 44:395–427
Visser AA, Kooij PW, Debets AJM et al (2011) Pseudoxylaria as stowaway of the fungus-growing termite nest: Interaction asymmetry between Pseudoxylaria, Termitomyces and free-living relatives. Fungal Ecol 4:322–332. https://doi.org/10.1016/j.funeco.2011.05.003
Visser AA, Nobre T, Currie CR et al (2012) Exploring the potential for Actinobacteria as defensive symbionts in fungus-growing termites. Microb Ecol 63:975–985. https://doi.org/10.1007/s00248-011-9987-4
Walker TN, Hughes WOH (2009) Adaptive social immunity in leaf-cutting ants. Biol Lett 5:446–448. https://doi.org/10.1098/rsbl.2009.0107
Wickham H (2009) Ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag, New York
Yanagawa A, Shimizu S (2007) Resistance of the termite, Coptotermes formosanus Shiraki to Metarhizium anisopliae due to grooming. BioControl 52:75–85. https://doi.org/10.1007/s10526-006-9020-x
Yanagawa A, Fujiwara-Tsujii N, Akino T et al (2011) Musty odor of entomopathogens enhances disease-prevention behaviors in the termite Coptotermes formosanus. J Invertebr Pathol 108:1–6. https://doi.org/10.1016/j.jip.2011.06.001
Yanagawa A, Fujiwara-Tsujii N, Akino T et al (2012) Odor aversion and pathogen-removal efficiency in grooming behavior of the termite Coptotermes formosanus. PLoS One 7:e47412. https://doi.org/10.1371/journal.pone.0047412
Yu DW (2001) Parasites of mutualisms. Biol J Linn Soc 72:529–546. https://doi.org/10.1006/bijl.2000.0514
Zachariah N, Das A, Murthy TG, Borges RM (2017) Building mud castles: perspectives from brick-laying termites. Sci Rep 7:4692
Zhang MM, Poulsen M, Currie CR (2007) Symbiont recognition of mutualistic bacteria by Acromyrmex leaf-cutting ants. ISME J 1:313–320
Acknowledgements
This research was funded by the Council for Scientific and Industrial Research (37(1561)/12/EMR-II), the Ministry of Environment, Forests & Climate Change, the Department of Biotechnology, and the Department of Science and Technology-FIST, Government of India. We are grateful to C Viraktamath and Rashmi Shanbhag for termite identifications, Yuvaraj Ranganathan for help in Random Forest analyses, Srinivasan Kasinathan and Aprajita Sharma for GC-MS work, Tejas Murthy for providing processed soil, Yathiraj Ganesh for field work, Sunitha Murray for administrative support and Mahua Ghara, Nirmal Borkar, Kavita Venkataramani and E S Anupriya for help in weighing.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
ESM 1
(DOC 401 kb)
Rights and permissions
About this article
Cite this article
Katariya, L., Ramesh, P.B., Gopalappa, T. et al. Fungus-Farming Termites Selectively Bury Weedy Fungi that Smell Different from Crop Fungi. J Chem Ecol 43, 986–995 (2017). https://doi.org/10.1007/s10886-017-0902-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-017-0902-4