Abstract
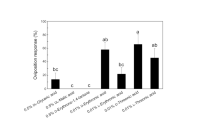
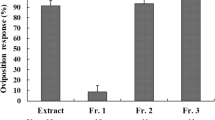
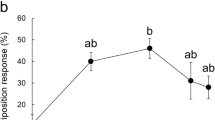
The common grass yellow butterfly, Eurema mandarina (formerly Eurema hecabe mandarina) (Lepidoptera, Pieridae), recently has been separated taxonomically from a subtropical population of Eurema hecabe in Japan. This species is widely distributed in the temperate region of Japan, and feeds mainly on various ligneous plants within the Fabaceae. We attempted to identify an oviposition stimulant for E. mandarina from its primary hosts, Albizia julibrissin and Lespedeza cuneata. In both hosts, crude extract and an aqueous fraction elicited oviposition responses from gravid females. A polar subfraction of the aqueous fraction also stimulated high oviposition-stimulatory activity, comparable to the original aqueous fraction, suggesting that E. mandarina females use water-soluble compounds for host recognition. Subsequent activity-directed fractionation by ion exchange chromatography indicated that one of the key substances was contained in the neutral/amphoteric fraction. Chemical analyses revealed that the active fractions of both hosts contained d-(+)-pinitol as the major component. We examined female responses to authentic d-pinitol and found that it induced oviposition responses at concentrations greater than 0.1 %. Since this cyclitol is omnipresent in Fabaceae, we conclude that d-pinitol plays a role in mediating oviposition of E. mandarina on fabaceous plants.





Similar content being viewed by others
References
Banno H (1984) The oviposition preference and larval food utilization in Eurema hecabe (Linnaeus) (Pieridae). Tyô to Ga 35:80–90
Braby MF (2005) Provisional checklist of genera of the pieridae (Lepidoptera: papilionoidea). Zootaxa 832:1–16
Braby MF, Trueman JWH (2006) Evolution of larval host plant associations and adaptive radiation in pierid butterflies. J Evol Biol 19:1677–1690
Braby MF, Vila R, Pierce NE (2006) Molecular phylogeny and systematics of the pieridae (Lepidoptera: papilionoidea): higher classification and biogeography. Zool J Linnean Soc 147:239–275
Chen JG, Dai G-H (2014) Effect of d-pinitol isolated and identified from Robinia pseudoacacia against cucumber powdery mildew. Sci Hortic 176:38–44
Chew FS, Renwick JAA (1995) Host plant choice in Pieris butterflies. In: Carde RT, Bell WJ (eds) Chemical ecology of insects 2. Chapman & Hall, New York, pp. 214–238
Chew FS, Robbins RK (1984) Egg-laying in butterflies. In: Vane-Wright RI, Ackery PR (eds) . The biology of butterflies Academic Press, London, pp. 65–79
Chiera JM, Streeter JG, Finer JJ (2006) Ononitol and pinitol production in transgenic soybean containing the inositol methyl transferase gene from Mesembryanthemum crystallinum. Plant Sci 171:647–654
Dittrich P, Brandl A (1987) Revision of the pathway of d-pinitol formation in Leguminosae. Phytochemistry 26:1925–1926
Dreyer DL, Binder RG, Chan BG, Waiss ACJR, Hartwig EE, Beland GL (1979) Pinitol, a larval growth inhibitor for Heliothis zea in soybeans. Experientia 35:1182–1183
Ehrlich PR (1958) The comparative morphology, phylogeny and higher classification of the butterflies (Lepidoptera: Papilionoidae). Univ Kansas Sci Bull 39:305–370
Ferrer-Paris JR, Sánchez-Mercado A, Viloria AL, Donaldson J (2013) Congruence and diversity of butterfly-host plant associations at higher taxonomic levels. PLoS One 8:e63570
Honda K (1990) Identification of host-pant chemicals stimulating oviposition by swallowtail butterfly, Papilio protenor. J Chem Ecol 16:325–337
Honda K (1995) Chemical basis of differential oviposition by lepidopterous insects. Arch Insect Biochem Physiol 30:1–23
Honda K, Nishii W, Hayashi N (1997) Oviposition stimulants for sulfur butterfly, Colias erate poliographys: Cyanoglucoside as synergists involved in host preference. J Chem Ecol 23:323–331
Honda K, Minematsu H, Muta K, Ômura H, Nishii W (2012) d-pinitol as a key oviposition stimulant for sulfur butterfly, Colias erate: chemical basis for female acceptance of host- and non-host plants. Chemoecology 22:55–63
Huang X, Renwick JAA (1993) Differential selection of host plants by two Pieris species: the role of oviposition stimulants and deterrents. Entomol Exp Appl 68:59–69
Huang X, Renwick JAA (1994) Relative activities of glucosinolates as oviposition stimulants for Pieris rapae and P. napi oleracea. J Chem Ecol 20:1025–1037
Huang X, Renwick JAA, Sachdev-Gupta K (1993) Oviposition stimulants and deterrents regulating differential acceptance of Iberis amara by Pieris rapae and P. napi oleracea. J Chem Ecol 19:1645–1663
Huang X, Renwick JAA, Sachdev-Gupta K (1994) Oviposition stimulants in Barbarea vulgaris for Pieris rapae and P. napi oleracea: isolation and differential activity. J Chem Ecol 20:423–438
Janz N, Nylin S (1998) Butterflies and plants: A phylogenetic study. Evolution 52:486–502
Kato Y (1999) Fringe colour, seasonal morph and host-plant use of the pierid butterfly, Eurema hecabe (L.) (Lepidoptera, Pieridae) on Okinawa-jima Island. Trans lepid Soc Jpn 50:111–121 (in Japanese with English summary)
Kato Y (2000a) Does mating occur among populations of two types in the butterfly Eurema hecabe (L.) (Lepidoptera, Pieridae)? Trans lepid Soc Jpn 52:63–66 (in Japanese with English summary)
Kato Y (2000b) Host-plant adaptation in two sympatric types of the butterfly Eurema hecabe (L.) (Lepidoptera: pieridae). Entomol Sci 3:459–463
Kato Y, Yata O (2005) Geographic distribution and taxonomical status of two types of Eurema hecabe (L.) (Lepidoptera, Pieridae) in south-western Japan and Taiwan. Trans lepid Soc Jpn 56:171–183 (in Japanese with English summary)
Kato Y, Hiroki M, Handa H (1992) Interpopulation variation in adaptation of Eurema hecabe (Lepidoptera, pieridae) to host plant. Jpn J Entomol 60:749–759
Kobayashi A, Hiroki M, Kato Y (2001) Sexual isolation between two sympatric types of the butterfly, Eurema hecabe (L.). J Insect Behav 14:353–362
Mithöfer A, Boland W (2012) Plant defense against herbivores: chemical aspects. Annu Rev Plant Biol 63:431–450
Narita S, Nomura M, Kato Y, Fukatsu T (2006) Genetic structure of sibling butterfly species affected by Wolbachia infection sweep: evolutionary and biogeographical implications. Mol Ecol 15:1095–1108
Narita S, Nomura M, Kato Y, Yata O, Kageyama D (2007) Molecular phylogeography of two sibling species of Eurema butterflies. Genetica 131:241–253
Negishi O, Mun’im A, Negishi Y (2015) Content of methylated inositols in familiar edible plants. J Agric Food Chem 63:2683–2688
Nishida R (2014) Chemical ecology of insect-plant interactions: ecological significance of plant secondary metabolites. Biosci Biotechnol Biochem 78:1–13
Nishida R, Fukami H (1989) Oviposition stimulants of an Aristolochiaceae-feeding swallowtail butterfly, Atrophaneura alcinous. J Chem Ecol 15:2565–2575
Nishida R, Ohsugi T, Kokubo S, Fukami H (1987) Oviposition stimulants of a Citrus-feeding swallowtail butterfly, Papilio xuthus L. Experientia 43:342–344
Numata A, Hokimoto K, Shimada A, Yamaguchi H, Takaishi K (1978) Feeding stimulants for the larvae of the yellow butterfly, Eurema hecabe mandarina (Lepidoptera: pieridae). Appl Entomol Zool 13:133–135
Numata A, Hokimoto K, Shimada A, Yamaguchi H, Takaishi K (1979) Plant constituents biologically active to insects. I. Feeding stimulants for the larvae of the yellow butterfly. Eurema hecabe mandarina. Chem Pharm Bull 27:602–608
Numata A, Yamaguchi H, Hokimoto K, Ohtani M, Takaishi K (1985) Host-plant selection by the yellow butterfly larvae, Eurema hecabe mandarina (Lepidoptera: pieridae): attractants and arrestants. Appl Entomol Zool 20:314–321
Obendorf RL, Sensenig EM, Wu J, Ohashi M, O’sullivan TE, Kosina SM, Schnebly SR (2008) Soluble carbohydrates in mature soybean seed after feeding d-chiro-inositol, myo-inositol, or d-pinitol to stem-leaf-pod explants of low-raffinose, low-stachyose lines. Plant Sci 175:650–655
Ohsugi T, Nishida R, Fukami H (1991) Multi-component system of oviposition stimulants for a Rutaceae-feeding swallowtail butterfly, Papilio xuthus (Lepidoptera: Papilionidae. Appl Entomol Zool 26:29–40
Papaj DR, Feeny P, Sachdev-Gupta K, Rosenberry L (1992) d-(+)-pinitol, an oviposition stimulant for the pipevine swallowtail butterfly, Battus philenor. J Chem Ecol 18:799–815
Phillips DV, Wilson DO, Dougherty DE (1984) Soluble carbohydrates in legumes and nodulated nonlegumes. J Agric Food Chem 32:1289–1291
Poongothai G, Sripathi SK (2013) A review of insulinomimetic pinitol from plants. Int. J Pharm Bio Sci 4:992–1009
Reese JC, Chan BG, Waiss ACJR (1982) Effects of cotton condensed tannin, maysin (corn) and pinitol (soybeans) on Heliothis zea growth and development. J Chem Ecol 8:1429–1436
Renwick JAA (2002) The chemical world of crucivores: lures, treats and traps. Entomol Exp Appl 104:35–42
Renwick JAA, Chew FS (1994) Oviposition behavior in Lepidoptera. Annu Rev Entomol 39:377–400
Renwick JAA, Radke CD, Sachdev-Gupta K (1989) Chemical constituents of Erysimum cheiranthoides deterring oviposition by the cabbage butterfly, Pieris rapae. J Chem Ecol 15:2161–2169
Renwick JAA, Radke CD, Sachdev-Gupta K, Städler E (1992) Leaf surface chemicals stimulating oviposition by Pieris rapae (Lepidoptera: pieridae) on cabbage. Chemoecology 3:33–38
Sachdev-Gupta K, Renwick JAA, Radke CD (1990) Isolation and identification of oviposition deterrents to cabbage butterfly, Pieris rapae, from Erysimum cheiranthoides. J Chem Ecol 16:1059–1067
Savela M (2014) Lepidoptera and some other life forms. www.nicfunetfi/pub/sci/bio/life/insecta/lepidoptera/ditrysia/papilionoidea/pieridae/coliadinae/ Accessed 16 Feb 2016
Streeter JG, Lohnes DG, Fioritto RJ (2001) Patterns of pinitol accumulation in soybean plants and relationships to drought tolerance. Plant Cell Environ 24:429–438
Thompson JN, Pellmyr O (1991) Evolution of oviposition behavior and host preference in Lepidoptera. Annu Rev Entomol 36:65–89
van Loon JJA, Blaakmer A, Griepink FC, van Beek TA, Schoonhoven LM, de Groot A (1992) Leaf surface compound from Brassica oleracea (Cruciferae) induces oviposition by Pieris brassicae (Lepidoptera: pieridae). Chemoecology 3:39–44
Wahlberg N, Braby MF, Brower AVZ, Jong R DE, Lee M-M, Nylin S, Pierce NE, Sperling FAH, Vila R, Warren AD, Zakharov E (2005) Synergistic effects of combining morphological and molecular data in resolving the phylogeny of butterflies and skippers. Proc R Soc B 272:1577–1586.
Wink M (2013) Evolution of secondary metabolites in legumes (Fabaceae). S Afr J Bot 89:164–175
Acknowledgments
The HR-MS and the optical rotation measurements were conducted using a Thermo Fischer LTQ Orbitrap XL hybrid mass spectrometer and a JASCO DIP-370 spectropolarimeter, respectively, at the Natural Science Center for Basic Research and Development (N-BARD), Hiroshima University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 190 kb)
Rights and permissions
About this article
Cite this article
Mukae, Sy., Ohashi, T., Matsumoto, Y. et al. d-Pinitol in Fabaceae: an Oviposition Stimulant for the Common Grass Yellow Butterfly, Eurema mandarina . J Chem Ecol 42, 1122–1129 (2016). https://doi.org/10.1007/s10886-016-0775-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-016-0775-y