Abstract
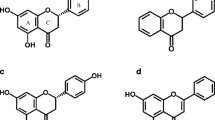
We synthesized 23 6-alkoxycoumarin derivatives, 20 of which are novel compounds. The structures of all compounds were confirmed by NMR, MS, and elemental analysis, and their antifeedant and termiticidal activities against Coptotermes formosanus Shiraki were examined. In a no-choice test, 6-(2-pentynyloxy)coumarin (2v), 6-(2-butynyloxy)coumarin (2u), 6-(2-octynyloxy)coumarin (2w), and 6-methoxycoumarin (2a), demonstrated high termiticidal activity at a concentration of 10 μmol. At a concentration of 5 μmol, 6-(2-butynyloxy)coumarin (2u) produced the highest mortality among the compounds tested. On the other hand, all of the 6-alkoxycoumarins showed antifeedant activity at both concentrations, except 6-octadecyloxycoumarin (2j) that was inactive at 5 μmol. Among the 23 compounds and the control, 6-ethoxycoumarin (2b), 6-isopropoxycoumarin (2d), and 6-isobutoxycoumarin (2f) exhibited the highest antifeedant activity with no mass loss (0.00%) at a concentration of 10 μmol. Our findings indicate that the presence of alkenyloxy and alkynyloxy groups was important for the termiticidal activity, while the incorporation of alkoxy groups with longer alkyl chains tended to reduce both the termiticidal and antifeedant activities. Furthermore, short chain alkoxy and arylalkoxy-substituted analogs showed good antifeedant activity, but methoxy groups on the benzene ring had a negative effect.




Similar content being viewed by others
References
Adfa, M., Koketsu, M. and Ebihara, M. 2010a. 6-Benzyloxycoumarin. Acta Cryst. E66:o2190.
Adfa, M., Yoshimura, T., Komura, K., and Koketsu, M. 2010b. Antitermite activities of coumarin derivatives and scopoletin from Protium javanicum Burm. f. J. Chem. Ecol. 36:720–726.
Arihara, S., Umeyama, A., Bando, S., Imoto, S., Ono, M., and Yoshikawa, K. 2004. Three new sesquiterpenes from the black heartwood of Cryptomeria japonica. Chem. Pharm. Bull. 52:463–465.
Culliney, T. W. and Grace, J. K. 2000. Prospects for the biological control of subterranean termites (Isoptera: Rhinotermitidae), with special reference to Coptotermes formosanus. Bull. Entomol. Res. 90:9–21.
Devi, K.K., Seth, N., Kothamasi, S., and Kothamasi, D. 2007. Hydrogen cyanide-producing rhizobacteria kill subterranean termite Odontotermes obesus (Rambur) by cyanide poisoning under in vitro conditions. Curr. Microbiol. 54:74–78.
Durst, G. L., Norman, B. H., Pfeifer, L. A., and Richardson, T. I. 2007. Substituted benzopyrans as selective estrogen receptor-beta agonists. Patent US 2007/0106082 A1.
Evans, T. A., and Gleeson, P. V. 2006. The effect of bait design on bait consumption in termites (Isoptera: Rhinotermitidae). Bull. Entomol. Res. 96:85–90.
Ganapaty, S., Thomas, P. S., Fotso, S., and Laatsch, H. 2004. Antitermitic quinones from Diospyros sylvatica. Phytochemistry 65:1265–1271.
Ishizuka, M., Tanikawa, T., Tanaka, K. D., Heewon, M., Okajima, F., Sakamoto, K. Q., and Fujita, S. 2008. Pesticide resistance in wild mammals—Mechanisms of anticoagulant resistance in wild rodents. J. Toxicol. Sci. 33:283–291.
Krutmuang, P., and Mekchay, S. 2005. Pathogenicity of entomopathogenic fungi Metarhizium anisopliae against termites. Paper of conference on international agricultural research for development, Stuttgart-Hohenhein, October 11–13, 2005. On line at. http://www.tropentag.de/2005/abstracts/full/355.pdf.
Meyer, J. R. 2005. Isoptera. Department of Entomology. NC State University. Online at. http://www.cals.ncsu.edu/course/ent425/compendium/termites.html.
Mo, J., Wang, Z., Song, X., Guo, J., Cao, X., and Cheng, J. 2006. Effects of sublethal concentration of ivermectin on behaviour of Coptotermes formasanus. Sociobiology 47:687–696.
Murray, R. D. H., Mendez, J., and Brown, S. A. 1982. The Natural Coumarins (Occurrence, Chemistry and Biochemistry). Wiley, New York.
Ohmura, W., Doi, S., Aoyama, M., and Ohara, S. 2000. Antifeedant activity of flavonoids and related compounds against the subterranean termite Coptotermes formosanus Shiraki. J. Wood Sci. 46:149–153.
Reyes-Chilpa, R., Viveros-Rodríguez, N., Gómez-Garibay, F., and Alavez-Solano, D. 1995. Antitermitic activity of Lonchocarpus castilloi flavonoids and heartwood extracts. J. Chem. Ecol. 21, 455–463.
Schönberg, A., and Latif, N. 1954. Furochromones and coumarins. XI. The molluscicidal activity of bergapten, isopimpinillin and xanthotoxin. J. Am. Chem. Soc. 76:6208.
Sekine, N., Ashitani, T., Murayama, T., Shibutani, S., Hattori, S., and Takahashi, K. 2009. Bioactivity of latifolin and its derivatives against termites and fungi. J. Agric. Food Chem. 57:5707–5712.
Serit, M., Ishida, M., Nakata, K., Kim, M., and Takahashi, S. 1992. Antifeedency potency of neem (Azadirachta indica) extractives and limonoids against termite (Reticulitermes speratus). J. Pesticide. Sci. 17: 267–273.
Shah, K. R., and Trivedi, K. N. 1975. Synthesis of forocoumarins. XXII. Synthesis of 7H-furo[3,2-f][1]benzopyran-7-one. J. Indian Chem. Soc. 52:436–437.
Soine, T. O. 1964. Naturally occurring coumarins and related physiological activities. J. Pharm. Sci. 53:231–264.
Takaishi, K., Izumi, M., Baba, N., Kawazu, K., and Nakajima, S. 2008. Synthesis and biological evaluation of alkoxycoumarins as novel nematicidal constituents. Bioorg. Med. Chem. Lett. 18:5614–5617.
Verma, M., Sharma, S., and Prasad, R. 2009. Biological alternatives for termite control: A review. Int. Biodeter. Biodegr. 63:959–972.
Weeks, B., and Baker, P. 2004. Subterranean termite (Isoptera: Rhinotermitidae) mortality due to entomopathogenic nematodes (Nematoda: Steinernematidae, Heterorhabditidae). University of Arizona College of Agriculture. 2004 Turfgrass and Ornamental Research Report. Online at http://www.cals.arizona.edu/pubs/crops/az1359/az13591b.pdf
Zhu, B. C. R., Henderson, G., Sauer, A. M., Yu, Y., Crowe, W., and Laine, R. A. 2003. Structure–activity of valencenoid derivatives and their repellence to the formosan subterranean termite. J. Chem. Ecol. 29:2695–2701.
Acknowledgements
We thank the Directorate General of Higher Education, Indonesia, for awarding the scholarship to Morina Adfa. We also thank to M. Ninomiya for measurement of the mass spectra. A part of this research was conducted under the Cooperative Study Program of the Deterioration Organisms Laboratory and Living-Sphere Simulation Field (DOL/LSF) of the Research Institute for Sustainable Humanosphere, Kyoto University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adfa, M., Hattori, Y., Yoshimura, T. et al. Antifeedant and Termiticidal Activities of 6-Alkoxycoumarins and Related Analogs Against Coptotermes formosanus Shiraki. J Chem Ecol 37, 598–606 (2011). https://doi.org/10.1007/s10886-011-9968-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-011-9968-6