Abstract
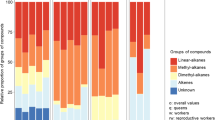
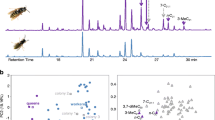
In most ants, bees, and wasps, the workers are capable of challenging the reproductive monopoly of the queen by laying unfertilized, male eggs. An important mechanism that can resolve this conflict is policing, whereby the queen or workers prevent successful worker reproduction by selectively eating worker-laid eggs or by attacking egg-laying workers. Egg policing by workers has been shown to occur in several social wasp species, but the information used by worker wasps to discriminate between queen-laid and worker-laid eggs has never been investigated. Our aim, therefore, was to investigate if hydrocarbons might be used in egg policing by workers in the common wasp, Vespula vulgaris, where worker policing previously has been shown to be effective. Our results show that 51 different hydrocarbons are present on the surface of newly-laid eggs, and that there are pronounced quantitative differences in the hydrocarbon profiles of queen-laid and worker-laid eggs, with longer-chained alkenes and methylated alkanes (C28–C31) in particular being more abundant on the surface of queen-laid eggs. We further show that the hydrocarbon profiles on the surface of queen-laid and worker-laid eggs resemble those found on the mother queen’s and workers’ cuticles. Interestingly, longer-chained methylated alkanes also were more abundant on the cuticle of both mother queens and reproductive workers, suggesting that these compounds are linked to fertility, as has also been found to be the case in several ant species.



Similar content being viewed by others
References
Aitchison, J. 1986. The Statistical Analysis of Compositional Data. Chapman and Hall, London.
Bagnères, A.-G. and Blomquist, G. J. 2010. Site of synthesis, mechanism of transport and selective deposition of hydrocarbons, pp. 75–99, in G. J. Blomquist and A.-G. Bagnères (eds.), Insect Hydrocarbons: Biology, Biochemistry, and Chemical Ecology. Cambridge University Press, Cambridge.
Billen, J. P. J. 1987. New structural aspects of the Dufour’s and venom glands in social insects. Naturwissenschaften 74:340–341.
Billen, J. 2006. Morphology and ultrastructure of the Dufour gland in workers of social wasps (Hymenoptera, Vespidae). Arthropod. Struct. Dev. 35:77–84.
Bloch, G., Borst, D. W., Huang, Z. Y., Robinson, G. E., Cnaani, J., and Hefetz, A. 2000. Juvenile hormone titers, juvenile hormone biosynthesis, ovarian development and social environment in Bombus terrestris. J. Insect Physiol. 46:47–57.
Bonavita-Cougourdan, A., Clément, J. L., and Lange, C. 1987. Nestmate recognition: The role of cuticular hydrocarbons in the ant Camponotus vagus Scop. J. Entomol. Sci. 22:1–10.
Bonavita-Cougourdan, A., Theraulaz, G., Bagnères, A.-G., Roux, M., Pratte, M., Provost, E., and Clément, J.-L. 1991. Cuticular hydrocarbons, social organization and ovarian development in a Polistine wasp: Polistes dominulus Christ. Comp. Biochem. Physiol. B 100:667–680.
Butts, D. P., Espelie, K. E., and Hermann, H. R. 1991. Cuticular hydrocarbons of four species of social wasps in the subfamily Vespinae—Vespa crabro L, Dolichovespula maculata (L), Vespula squamosa (Drury) and Vespula maculifrons (Buysson). Comp. Biochem. Physiol. B 99:87–91.
Dani, F. R., Morgan, E. D., and Turillazzi, S. 1996. Dufour gland secretion of Polistes wasp: Chemical composition and possible involvement in nestmate recognition (Hymenoptera: vespidae). J. Insect Physiol. 42:541–548.
Dapporto, L., Dani, F. R., and Turillazzi, S. 2007. Social dominance molds cuticular and egg chemical blends in a paper wasp. Curr. Biol. 17:R504–R505.
de Biseau, J. C., Passera, L., Daloze, D., and Aron, S. 2004. Ovarian activity correlates with extreme changes in cuticular hydrocarbon profile in the highly polygynous ant, Linepithema humile. J. Insect Physiol. 50:585–593.
D’Ettorre, P., Heinze, J., and Ratnieks, F. L. W. 2004a. Worker policing by egg-eating in the ponerine ant Pachycondyla inversa. Proc. R. Soc. Lond. B Biol. Sci. 271:1427–1434.
D’Ettorre, P., Heinze, E., Schulz, C., Francke, W., and Ayasse, M. 2004b. Does she smell like a queen? Chemoreception of a cuticular hydrocarbon signal in the ant Pachycondyla inversa. J. Exp. Biol. 207:1085–1091.
Dietemann, V., Peeters, C., Liebig, J., Thivet, V., and Hölldobler, B. 2003. Cuticular hydrocarbons mediate discrimination of reproductives and nonreproductives in the ant Myrmecia gulosa. Proc. Natl. Acad. Sci. U. S. A. 100:10341–10346.
Endler, A., Liebig, J., Schmitt, T., Parker, J. E., Jones, G. R., Schreier, P., and Hölldobler, B. 2004. Surface hydrocarbons of queen eggs regulate worker reproduction in a social insect. Proc. Natl. Acad. Sci. U. S. A. 101:2945–2950.
Endler, A., Liebig, J., and Hölldobler, B. 2006. Queen fertility, egg marking and colony size in the ant Camponotus floridanus. Behav. Ecol. Sociobiol. 59:490–499.
Fan, Y. L., Chase, J., Sevala, V. L., and Schal, C. 2002. Lipophorin-facilitated hydrocarbon uptake by oocytes in the German cockroach Blattella germanica (L.). J. Exp. Biol. 205:781–790.
Foster, K. R. and Ratnieks, F. L. W. 2001. Convergent evolution of worker policing by egg eating in the honeybee and common wasp. Proc. R. Soc. Lond. B 268:169–174.
Heinze, J., Stengl, B., and Sledge, M. F. 2002. Worker rank, reproductive status and cuticular hydrocarbon signature in the ant, Pachycondyla cf. inversa. Behav. Ecol. Sociobiol. 52:59–65.
Hölldobler, B. and Wilson, E. O. 2009. The Superorganism: The Beauty, Elegance, and Strangeness of Insect Societies. W.W. Norton & Company, New York.
Holman, L., Dreier, S., and D’Ettorre, P. 2010a. Selfish strategies and honest signalling: Reproductive conflicts in ant queen associations. Proc. R. Soc. B 277:2007–2015.
Holman, L., Jorgensen, C. G., Nielsen, J., and D’Ettorre, P. 2010b. Identification of an ant queen pheromone regulating worker sterility. Proc. R. Soc. B 277:3793–3800.
Hora, R. R., Ionescu-Hirsh, A., Simon, T., Delabie, J., Robert, J., Fresneau, D., and Hefetz, A. 2008. Postmating changes in cuticular chemistry and visual appearance in Ectatomma tuberculatum queens (Formicidae: Ectatomminae). Naturwissenschaften 95:55–60.
Howard, R. W., Perez-Lachaud, G., and Lachaud, J. P. 2001. Cuticular hydrocarbons of Kapala sulcifacies (Hymenoptera: Eucharitidae) and its host, the ponerine ant Ectatomma ruidum (Hymenoptera: Formicidae). Ann. Entomol. Soc. Am. 94:707–716.
Huang, Z. Y., Robinson, G. E., and Borst, D. W. 1994. Physiological correlates of division of labor among similarly aged honey bees. J. Comp. Physiol. A. 174:731–739.
Katzav-Gozansky, T., Soroker, V., Hefetz, A., Cojocaru, M., Erdmann, D. H., and Francke, W. 1997. Plasticity of caste-specific Dufour’s gland secretion in the honey bee (Apis mellifera L). Naturwissenschaften 84:238–241.
Katzav-Gozansky, T., Soroker, V., Ibarra, F., Francke, W., and Hefetz, A. 2001. Dufour’s gland secretion of the queen honeybee (Apis mellifera): An egg discriminator pheromone or a queen signal? Behav. Ecol. Sociobiol. 51:76–86.
le Conte, Y. and Hefetz, A. 2008. Primer pheromones in social Hymenoptera. Annu. Rev. Entomol. 53:523–542.
Lengyel, F., Westerlund, S. A., and Kaib, M. 2007. Juvenile hormone III influences task-specific cuticular hydrocarbon profile changes in the ant Myrmicaria eumenoides. J. Chem. Ecol. 33:167–181.
Liebig, J. 2010. Hydrocabon profiles indicate fertility and dominance status in ant, bee, and wasp colonies, pp. 254–281, in G. J. Blomquist and A.-G. Bagnères (eds.), Insect Hydrocarbons: Biology, Biochemistry, and Chemical Ecology. Cambridge University Press, Cambridge.
Lommelen, E., Johnson, C. A., Drijfhout, F. P., Billen, J., and Gobin, B. 2008. Egg marking in the facultatively queenless ant Gnamptogenys striatula: The source and mechanism. J. Insect Physiol. 54:727–736.
Martin, S. J., Jones, G. R., Châline, N., Middleton, H., and Ratnieks, F. L. W. 2002. Reassessing the role of the honeybee (Apis mellifera) Dufour’s gland in egg marking. Naturwissenschaften 89:528–532.
Martin, S. J., Jones, G. R., Châline, N., and Ratnieks, F. L. W. 2004. Role of hydrocarbons in egg recognition in the honeybee. Physiol. Entomol. 29:395–399.
Meunier, J., Delemont, O., and Lucas, C. 2011. Recognition in ants: Social origin matters. Plos One 6.
Mitra, A. and Gadagkar, R. 2011. Can Dufour’s gland compounds honestly signal fertility in the primitively eusocial wasp Ropalidia marginata? Naturwissenschaften 98:157–161.
Mitra, A., Saha, P., Chaoulideer, M. E., Bhadra, A., and Gadagkar, R. 2011. Chemical communication in Ropalidia marginata: Dufour’s gland contains queen signal that is perceived across colonies and does not contain colony signal. J. Insect Physiol. 57:280–284.
Monnin, T. 2006. Chemical recognition of reproductive status in social insects. Ann. Zool. Fenn. 43:515–530.
Monnin, T. and Peeters, C. 1997. Cannibalism of subordinates’ eggs in the monogynous queenless ant Dinoponera quadriceps. Naturwissenschaften 84:499–502.
Queller, D. C. and Strassmann, J. E. 2009. Beyond society: The evolution of organismality. Phil. Trans. R. Soc. Lond. B 364:3143–3155.
Ratnieks, F. L. W. 1995. Evidence for a queen-produced egg-marking pheromone and its use in worker policing in the honey-bee. J. Apic. Res. 34:31–37.
Ratnieks, F. L. W. and Visscher, P. K. 1989. Worker policing in the honeybee. Nature 342:796–797.
Ratnieks, F. L. W. and Wenseleers, T. 2008. Altruism in insect societies and beyond: Voluntary or enforced? Trends Ecol. Evol. 23:45–52.
Ratnieks, F. L. W., Foster, K. R., and Wenseleers, T. 2006. Conflict resolution in insect societies. Annu. Rev. Entomol. 51:581–608.
Schal, C., Sevala, V. L., Capurro, M. L., Snyder, T. E., Blomquist, G. J., and Bagnères, A.-G. 2001. Tissue distribution and lipophorin transport of hydrocarbons and sex pheromones in the house fly, Musca domestica. J. Insect Sci. 1:1–12.
Smith, A. A., Holldobler, B., and Liebig, J. 2008. Hydrocarbon signals explain the pattern of worker and egg policing in the ant Aphaenogaster cockerelli. J. Chem. Ecol. 34:1275–1282.
Smith, A. A., Holldober, B., and Liebig, J. 2009. Cuticular hydrocarbons reliably identify cheaters and allow enforcement of altruism in a social insect. Curr. Biol. 19:78–81.
Strassmann, J. E. and Queller, D. C. 2010. The social organism: Congresses, parties, and committees. Evolution 64:605–616.
van Zweden, J. S., Heinze, J., Boomsma, J. J., and D’Ettorre, P. 2009. Ant queen egg-marking signals: Matching deceptive laboratory simplicity with natural complexity. PLoS One 4:4718.
Wenseleers, T. and Ratnieks, F. L. W. 2006. Comparative analysis of worker reproduction and policing in eusocial Hymenoptera supports relatedness theory. Am. Nat. 168:E163–E179.
Acknowledgments
We thank the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen), and the Research Foundation Flanders (grant no. GNM-B5996-KAN2006) for funding. Patrizia d’Ettorre was supported by an EU Marie Curie Excellence Grant CODICES-EXT-CT-2004-014202.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
Hydrocarbon composition (% ± SD) of the surface of queen-laid (Q-laid) and worker-laid (W-laid) eggs and of the cuticle of reproductive queens (Q), spring-collected queens (SQ), virgin queens (VQ), and workers with fully-developed (FD), partially developed (PD), and undeveloped ovaries (UND) in Vespula vulgaris. For dimethyl alkanes, x represents the position of the second methyl group. For instance, for peak 6, x = 7, 9, 11, and 13 is used to denote that peak 6 was composed of a mixture of 5,7-, 5,9-, 5,11-, and 5,13-diMeC23. For peaks 45, 46, and 48, the positions of the methyl groups were not identifiable (DOCX 29 kb)
Table S2
Factor loadings of the hydrocarbon compounds on the six PCs with an eigenvalue greater than 1. For information on the value of x, see Table S1 (DOCX 24 kb)
Table S3
Classification matrix showing the correct classification of queen-laid (Q-laid) and worker-laid (W-laid) eggs in Vespula vulgaris based on the cuticular hydrocarbons on the egg surface (DOCX 18 kb)
Table S4
Classification matrix showing the correct classification of reproductive queens (Q), spring-collected queens (SQ), virgin queens (VQ), and workers with fully-developed (FD), partially developed (PD), and undeveloped ovaries (UND) in Vespula vulgaris based on their cuticular hydrocarbon profiles (DOCX 19 kb)
Table S5
Classification matrix showing the correct classification of workers to their colony of origin in Vespula vulgaris based on their cuticular hydrocarbon profiles (DOCX 18 kb)
Table S6
Factor loadings of the hydrocarbon compounds on the seven PCs with an eigenvalue greater than 1. For information on the value of x, see Table S1 (DOCX 24 kb)
Table S7
Classification matrix showing the correct classification of workers with undeveloped ovaries to their colony of origin in Vespula vulgaris based on their cuticular hydrocarbon profiles (DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Bonckaert, W., Drijfhout, F.P., d’Ettorre, P. et al. Hydrocarbon Signatures of Egg Maternity, Caste Membership and Reproductive Status in the Common Wasp. J Chem Ecol 38, 42–51 (2012). https://doi.org/10.1007/s10886-011-0055-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-011-0055-9