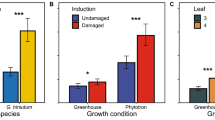
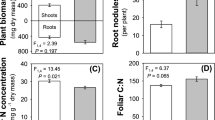
Abstract
Plants respond to insect herbivory by producing dynamic changes in an array of defense-related volatile and nonvolatile secondary metabolites. A scaled response relative to herbivory levels and nutrient availability would be adaptive, particularly under nutrient-limited conditions, in minimizing the costs of expressed defensive pathways and synthesis. In this study, we investigated effects of varying nitrogen (N) fertilization (42, 112, 196, and 280 ppm N) on levels of cotton plant (Gossypium hirsutum) phytohormones [jasmonic acid (JA) and salicylic acid (SA)], terpenoid aldehydes (hemigossypolone, heliocides H1, H2, H3, and H4), and volatile production in response to beet armyworm (Spodoptera exigua) herbivory. Additional bioassays assessed parasitoid (Cotesia marginiventris) host-searching success in response to cotton plants grown under various N fertilizer regimes. At low N input (42 ppm N), herbivore damage resulted in significant increases in local leaf tissue concentrations of JA and volatiles and in systemic accumulation of terpenoid aldehydes. However, increased N fertilization of cotton plants suppressed S. exigua-induced plant hormones and led to reduced production of various terpenoid aldehydes in damaged mature leaves and undamaged young leaves. While increased N fertilization significantly diminished herbivore-induced leaf volatile concentrations, the parasitism of S. exigua larvae by the parasitoid C. marginiventris in field cages did not differ among N treatments. This suggests that, despite significant N fertilization effects on herbivore-induced plant defenses, at short range, the parasitoids were unable to differentiate between S. exigua larvae feeding on physiologically different cotton plants that share large constitutive volatile pools releasable when damaged by herbivores.

Similar content being viewed by others
References
Alborn, H. T., Röse, U. S. R., and McAuslane, H. J. 1996. Systemic induction of feeding deterrents in cotton plants by feeding of Spodoptera spp. larvae. J. Chem. Ecol. 22:919–932.
Berenbaum, M. R. 1995. The chemistry of defense: theory and practice. Proc. Natl. Acad. Sci. U.S.A. 92:2–8.
Bezemer, T. M., Wagenaar, R., van Dam, N. M., and Wäckers, F. L. 2003. Interactions between above- and belowground insect herbivores as mediated by the plant defense system. Oikos 101:555–562.
Bezemer, T. M., Wagenaar, R., van Dam, N. M., van der Putten, W. H., and Wäckers, F. L. 2004. Above- and below-ground terpenoid aldehyde induction in cotton, Gossypium herbaceum, following root and leaf injury. J. Chem. Ecol. 30:53–67.
Browse, J., and Howe, G. A. 2008. New weapons and a rapid response against insect attack. Plant Physiol. 146:832–838.
Chen, Y., Ruberson, J. R., and Olson, D. 2008. Nitrogen fertilization rate affects feeding, larval performance, and oviposition preference of the beet armyworm, Spodoptera exigua, on cotton. Entomol. Exp. Appl. 126:245–255.
Chen, Y., Ruberson, J. R., Lewis, W. J., and Bednarz, C. 2006. Herbivore feeding and induction of systemic resistance in cotton plants, pp. 1510–1520, in Proceedings, Beltwide Cotton Conferences, National Cotton Council, Memphis, Tennessee.
Choh, Y., Shimoda, T., Ozawa, R., Dicke, M., and Takabayashi, J. 2004. Exposure of lima bean leaves to volatiles from herbivore-induced conspecific plants results in emission of carnivore attractants: active or passive process? J. Chem. Ecol. 30:1305–1317.
Cipollini, D. F., and Bergelson, J. 2001. Plant density and nutrient availability constrain constitutive and wound-induced expression of trypsin inhibitors in Brassica napus. J. Chem. Ecol. 27:593–610.
Coviella, C. E., Stipanovic, R. D., and Trumble, J. T. 2002. Plant allocation to defensive compounds: interactions between elevated CO2 and nitrogen in transgenic cotton plants. J. Exp. Bot. 53:323–331.
De Moraes, C. M., Lewis, W. J., Paré, P. W., Alborn, H. T., and Tumlinson, J. H. 1998. Herbivore-infested plants selectively attract parasitoids. Nature 393:570–573.
Dicke, M., Sabelis, M. W., Takabayashi, J., Bruin, J., and Posthumus, M. A. 1990. Plant strategies of manipulating predator–prey interactions through allelochemicals: prospects for application in pest control. J. Chem. Ecol. 16:3091–3118.
Dudt, J. F., and Shure, D. J. 1994. The influence of light and nutrients on foliar phenolics and insect herbivory. Ecology 75:86–98.
Duffy, S. S., and Stout, M. J. 1996. Antinutritive and toxic components of plant defense against insects. Arch. Insect Biochem. Physiol. 32:3–37.
Eller, J. J., Tumlinson, J. H., and Lewis, W. J. 1988. Beneficial arthropod behavior mediated by airborne semiochemicals. II. Olfactometric studies of host location by the parasitoid Microplitis croceipes (Cresson) (Hymenoptera: Braconidae). J. Chem. Ecol. 14:425–434.
Elliger, C. A., Chan, B. G., and Waiss, A. C. Jr. 1978. Relative toxicity of minor cotton terpenoids compared to gossypol. J. Econ. Entomol. 71:161–164.
Elzen, G. W., Williams, H. J., Bell, A. A., Stipanovic, R. D., and Vinson, S. B. 1985. Quantification of volatile terpenes of glanded and glandless Gossypium hirsutum cultivars and lines by gas chromatography. J. Agric. Food. Chem. 33:1079–1082.
Engelberth, J., Schmelz, E. A., Alborn, H. T., Cardoza, Y. J., Huang, J., and Tumlinson, J. H. 2003. Simultaneous quantification of jasmonic acid and salicylic acid in plants by vapor phase extraction and gas chromatography-chemical ionization-mass spectrometry. Analyt. Biochem. 321:242–250.
Firn, R. D., and Jones, C. G. 2006. Do we need a new hypothesis to explain plant VOC emissions? Trends Plant Sci. 11:112–113.
Gouinguené, S. P., and Turlings, T. C. J. 2002. The effects of abiotic factors on induced volatile emission in corn plants. Plant Physiol. 129:1296–1307.
Gouinguené, S., Pickett, J. A., Wadhams, L. J., Birkett, M. A., and Turlings, T. C. J. 2005. Antennal electrophysiological responses of three parasitic wasps to caterpillar-induced volatiles from maize (Zea mays mays), cotton (Gossypium herbaceum), and cowpea (Vigna unguiculata). J. Chem. Ecol. 31:1023–1038.
Hemming, J. D. C., and Lindroth, R. L. 1999. Effects of light and nutrient availability on aspen: growth, phytochemistry, and insect performance. J. Chem. Ecol. 25:1687–1714.
Hoballah, M. E. F., Tamò, C., and Turlings, T. C. J. 2002. Differential attractiveness of induced odors emitted by eight maize varieties for the parasitoid Cotesia marginiventris: is quality or quantity important? J. Chem. Ecol. 28:951–968.
Krischik, V. A., and Denno, R. F. 1983. Individual, population, and geographic patterns in plant defense, pp. 463–512, in R. F. Denno, and M. S. McClure (eds.). Variable Plants and Herbivores in Natural and Managed SystemsAcademic, New York, New York, USA.
Lou, Y., and Baldwin, I. T. 2004. Nitrogen supply influences herbivore-induced direct and indirect defenses and transcriptional responses in Nicotiana attenuata. Plant Physiol. 135:496–506.
Loughrin, J. H., Manukian, A., Heath, R. R., Turlings, T. C. J., and Tumlinson, J. H. 1994. Diurnal cycle of emission of induced volatile terpenoids by herbivore-injured cotton plant. Proc. Natl. Acad. Sci. U.S.A. 91:11836–11840.
Loughrin, J. H., Manukian, A., Heath, R. R., and Tumlinson, J. H. 1995. Volatiles emitted by different cotton varieties damaged by feeding beet armyworm larvae. J. Chem. Ecol. 21:1217–1227.
Matsui, K. 2006. Green leaf volatiles: hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 9:274–280.
McAuslane, H. J., and Alborn, H. T. 1998. Systemic induction of allelochemicals in glanded and glandless isogenic cotton by Spodoptera exigua feeding. J. Chem. Ecol. 24:399–416.
McAuslane, H. J., Alborn, H. T., and Toth, J. P. 1997. Systemic induction of terpenoid aldehydes in cotton pigment glands by feeding of larval Spodoptera exigua. J. Chem. Ecol. 23:2861–2879.
McKey, D. 1974. Adaptive patterns in alkaloid physiology. Am. Nat. 108:305–320.
McKey, D. 1979. The distribution of secondary compounds within plants, pp. 55–133, in G. A. Rosenthal, and D. H. Janzen (eds.). Herbivores: Their Interaction with Secondary Plant MetabolitesAcademic, New York, New York, USA.
McNeill, S., and Southwood, T. R. E. 1978. The role of nitrogen in the development of insect/plant relationships, pp. 77–98, in J. S. Harborne (ed.). Aspects of Plant and Animal CoevolutionAcademic, London.
Niki, T., Mitsuhara, I., Seo, S., Ohtsubo, N., and Ohashi, Y. 1998. Antagonistic effect of salicylic acid and jasmonic acid on the expression of pathogenesis-related (PR) protein genes in wounded mature tobacco leaves. Plant Cell Physiol. 39:500–507.
Nordlund, D. A., Jones, R. L., and Lewis, W. J. 1981. Semiochemicals, Their Role in Pest Control. Wiley, New York, USA.
Ohnmeiss, T., and Baldwin, I. T. 2000. Optimal defense theory predicts the ontogeny of an induced nicotine defense. Ecology 81:1765–1783.
Opitz, S., Kunert, G., and Gershenzon, J. 2008. Increased terpenoid accumulation in cotton (Gossypium hirsutum) foliage is a general wound response. J. Chem. Ecol. 34:508–522.
Pena-Cortés, H., Albrecht, T., Prat, S., Weiler, E. W., and Willmitzer, L. 1993. Aspirin prevents wound-induced gene expression in tomato leaves by blocking jasmonic acid biosynthesis. Planta 191:123–128.
Peñuelas, J., and Llusià, J. 2004. Plant VOC emissions: making use of the unavoidable. Trends Ecol. Evol. 19:402–404.
Pichersky, E., Sharkey, T. D., and Gershenzon, J. 2006. Plant volatiles: a lack of function or a lack of knowledge? Trends Plant Sci. 11:421.
Pickett, J. 1999. Insect-Plant Interactions and Induced Plant Defence. Wiley, New York, USA.
Reinbothe, S., Mollenhauer, B., and Reinbothe, C. 1994. JIPs and RIPs: the regulation of plant gene expression by jasmonates in response to environmental cues and pathogens. Plant Cell 6:1197–1209.
Röse, U. S. R., Lewis, W. J., and Tumlinson, J. H. 1998. Specificity of systemically released cotton volatiles as attractants for specialist and generalist parasitic wasps. J. Chem. Ecol. 24:303–319.
Sas Institute 1999. SAS/STAT User’s guide, version. 8th edn.SAS Institute, Inc., Cary, NC.
Schmelz, E. A., Alborn, H. A., Engelberth, J., and Tumlinson, J. H. 2003a. Nitrogen deficiency increases volicitin-induced volatile emission, jasmonic acid accumulation, and ethylene sensitivity in maize. Plant Physiol. 133:295–306.
Schmelz, E. A., Alborn, H. T., Banchio, E., and Tumlinson, J. H. 2003b. Quantitative relationship between induced jasmonic acid levels and volatile emission in Zea mays during Spodoptera exigua herbivory. Planta 216:665–673.
Schmelz, E. A., Engelberth, J., Alborn, H. T., O’Donnell, P., Sammons, M., Toshima, H., and Tumlinson, J. H. 2003c. Simultaneous analysis of phytohormones, phytotoxins, and volatile organic compounds in plants. Proc. Natl. Acad. Sci. U.S.A. 100:10552–10557.
Schmelz, E. A., Engelberth, J., Tumlinson, J. H., Block, A., and Alborn, H. T. 2004. The use of vapor phase extraction in metabolic profiling of phytohormones and other metabolites. Plant J. 39:790–808.
Stiling, P., and Moon, D. C. 2005. Quality or quantity: the direct and indirect effects of host plants on herbivores and their natural enemies. Oecologia 142:413–420.
Stipanovic, R. D., Altman, D. W., Begin, D. L., Greenblatt, G. A., and Benedict, J. H. 1988. Terpenoid aldehydes in upland cottons: analysis by aniline and HPLC methods. J. Agric. Food Chem. 36:509–515.
Stout, M. J., Brovont, R. A., and Duffey, S. S. 1998. Effects of nitrogen availability on expression of constitutive and inducible chemical defenses in tomato, Lycopersicon esculentum. J. Chem Ecol. 24:945–963.
Turlings, T. C. J., Tumlinson, J. H., Heath, R. R., Proveaux, A. T., and Doolittle, R. E. 1991a. Isolation and identification of allelochemicals that attract the larval parasitoid, Cotesia marginiventris (Cresson), to the microhabitat of one of its hosts. J. Chem. Ecol. 17:2235–2251.
Turlings, T. C. J., Tumlinson, J. H., Eller, F. J., and Lewis, W. J. 1991b. Larval-damaged plants: sources of volatile synomones that guide the parasitoid Cotesia marginiventris to the micro-habitat of its hosts. Entomol. Exp. Appl. 58:75–82.
Van Wassenhove, F. A., Dirinck, P. J., Schamp, N. M., and Vulsteke, G. A. 1990. Effects of nitrogen fertilizers on celery volatiles. J. Agric. Food Chem. 38:220–226.
Wäckers, F. L., and Bonifay, C. 2004. How to be sweet? Extrafloral nectar allocation by Gossypium hirsutum fits optimal defense theory predictions. Ecology 85:1512–1518.
Walling, L. L. 2000. The myriad plant responses to herbivores. J. Plant Growth Regul. 19:195–216.
Weissbecker, B., Van Loon, J. J. A., and Dicke, M. 1999. Electroantennogram responses of a predator, Perillus bioculatus, and its prey, Lepinotarsa decemlineata, to plant volatiles. J. Chem. Ecol. 25:2313–2325.
Wu, J., Hettenhausen, C., Schuman, M. C., and Baldwin, I. T. 2008. A comparison of two Nicotiana attenuata accessions reveals large differences in signaling induced by oral secretions of the specialist herbivore Manduca sexta. Plant Physiol. 146:927–939.
Acknowledgements
The research was supported by the Georgia Cotton Commission, Cotton Incorporated, and Grant-In-Aid of Research from the National Academy of Sciences, administered by Sigma Xi, the scientific Research Society to Y. Chen.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Y., Schmelz, E.A., Wäckers, F. et al. Cotton Plant, Gossypium hirsutum L., Defense in Response to Nitrogen Fertilization. J Chem Ecol 34, 1553–1564 (2008). https://doi.org/10.1007/s10886-008-9560-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-008-9560-x