Abstract
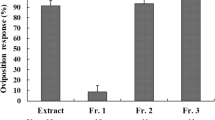
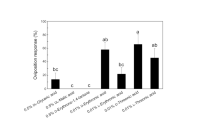
Elaphoside-A [p-vinylphenyl (β-d-glucopyranosyl)-(1→3)-β-d-allopyranoside], a Mediterranean fruit fly oviposition deterrent, was previously isolated from an Argentine collection of the fern Elaphoglossum piloselloides. In order to establish the structural requirements for the observed oviposition inhibition, we synthesized and characterized 4 known and 21 new aromatic glycosides structurally related to elaphoside-A. Their effects on the oviposition behavior of Ceratitis capitata females are discussed.



Similar content being viewed by others
References
Burger, K., Kluge, M., Koksch, B., Fehn, S., Böttcher, C., Hennig, L., and Müller, G. 2004. Hexafluoroacetone as protecting and activating reagent: a new approach to O-glycosides. Heterocycles. 64:143–52.
Cannizzaro, C. E., Thiel, I. M. E., and D’Accorso, N. B. 1998. Synthesis and debenzoylation products of two perbenzoylated 2-substituted 5-d-galactosyl-1,3,4-oxadiazoles. J. Heterocyclic Chem. 35:481–4.
Clingman, A. L. 1964. p-Vinylphenyl glycosides of cellobiose and maltose. J. Med. Chem. 7:242–3.
Conchie, J., Levvy, G. A., and Marsh, C. A. 1957. Methyl and phenyl glycosides of the common sugars. Adv. Carbohydr. Chem. 12:157–87.
Helferich, B., and Höfmann, H. J. 1952. p-Oxy-styrol-β-D-glucosid. Chem. Ber. 85:175–80.
Koschier, E. H., and Sedy, K. A. 2003. Labiate essential oils affecting host selection and acceptance of Thrips tabaci lindeman. Crop Prot. 22:929–34.
Kulkarni, M. M., Nagasampagi, B. A., Deshpande, S. G., and Sharma, R. N. 1987. Five chromenes from Blepharispermum subsessile. Phytochemistry 26:2969–71.
Ojika, M., Wakamatsu, K., Niwa, H., Yamada, K., and Hirono, I. 1984. Isolation and structures of two new p-hydroxystyrene glycosides, ptelatoside-A and ptelatoside-B from bracken, Pteridium aquilinum var. latiusculum, and synthesis of ptelatoside-A. Chem. Lett. 3:397–400.
Prajapati, V., Tripathi, A. K., Aggarwal, K. K., and Khanuja, S. P. S. 2005. Insecticidal, repellent and oviposition-deterrent activity of selected essential oils against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus. Bioresour. Technol. 96:1749–57.
Socolsky, C., Salvatore, A., Asakawa, Y., and Bardón, A. 2003. Bioactive new bitter-tasting p-hydroxystyrene glycoside and other constituents from the fern Elaphoglossum spathulatum. Arkivoc 2003:347–55.
Szentesi, A., Greany, P. D., and Chambers, D. L. 1979. Oviposition behavior of laboratory-reared and wild Caribbean fruit flies (Anastrepha suspensa; Diptera; Tephritidae): I. Selected chemical influences. Entomol. Exp. & Appl. 26:227–38.
Acknowledgments
We thank Ms. Y. Okamoto from Tokushima Bunri University for measuring the HR-MS spectra, Dr. M. Tanaka (TBU) for recording some of the NMR spectra, and Mr. J. Caram for drawing Fig. 2. C. Socolsky thanks Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) for a grant. Dr. N. D’Accorso is a research member of CONICET. This work was supported in part by a Grant-in-Aid for Scientific Research (B) N° 14403014 from the Ministry of Education, Science, Sports and Culture (Japan), CONICET, ANPCyT (project 06-13922), Universidad de Buenos Aires (UBA), and Consejo Nacional de Investigaciones de la Universidad Nacional de Tucumán (CIUNT), Argentina.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
p-Ethylphenyl 2,3,4,6-Tetra-O-Acetyl-α-d-Galactopyranoside (1)
High-resolution fast atom bombardment mass spectrum (HR-FAB-MS) m/z: 475.1599 [M + Na]+, calculated for C22H28O10Na: 475.1580; [α]D +251.8 (CHCl3, c 1.0 g/dl, 19.4°C); IR ν max [cm−1] (neat), 1,747, 1,608, 1,510, 1,371, 1,217, 1,070; 1H NMR (500 MHz, CDCl3), δ 6.98 (d, J = 8.8 Hz, H-2′, H-6′), 7.13 (d, J = 8.5 Hz, H-3′, H-5′), 2.60 (q, J = 7.5 Hz, H-1″), 1.21 (t, J = 7.5 Hz, H-2″), 5.73 (d, J = 3.6 Hz, H-1), 5.28 (dd, J = 10.9, 3.7 Hz, H-2), 5.58 (dd, J = 11.0, 3.4 Hz, H-3), 5.53 (dd, J = 3.4, 1.1 Hz, H-4), 4.37 (t, J = 6.6 Hz, H-5), 4.13 (dd, J = 11.2, 6.3 Hz, H-6a), 4.07 (dd, J = 11.2, 7.2 Hz, H-6b), 2.16, 2.07, 2.02, 1.94 (s, CH 3C=O); 13C NMR (125 MHz, CDCl3): δ 154.4 (C-1′), 116.8 (C-2′, C-6′), 128.9 (C-3′, C-5′), 139.0 (C-4′), 28.1 (C-1″), 15.8 (C-2″), 95.1 (C-1), 67.9 (C-2), 67.6 (C-3), 68.0 (C-4), 67.1 (C-5), 61.5 (C-6), 170.4, 170.3, 170.2, 170.0 (C=O), 20.74, 20.69, 20.64, 20.58 (CH3C=O).
p-Ethylphenyl 2,3,4,6-Tetra-O-Acetyl-α-d-Glucopyranoside (2)
High-resolution chemical ionization mass spectrum (HR-CI-MS) m/z: 453.1775 [M + H]+, calculated for C22H29O10: 453.1761; 1H NMR (500 MHz, CDCl3): δ 7.00 (d, J = 8.6 Hz, H-2′, H-6′), 7.13 (d, J = 8.4 Hz, H-3′, H-5′), 2.60 (q, J = 7.6 Hz, H-1″), 1.21 (t, J = 7.6 Hz, H-2″), 5.70 (d, J = 3.8 Hz, H-1), 5.03 (dd, J = 10.2, 3.6 Hz, H-2), 5.70 (t, J = 9.8 Hz, H-3), 5.15 (t, J = 9.9 Hz, H-4), 4.14 (ddd, J = 10.3, 4.5, 2.2 Hz, H-5), 4.25 (dd, J = 12.3, 4.6 Hz, H-6a), 4.06 (dd, J = 12.3, 2.3 Hz, H-6b), 2.06, 2.05, 2.04, 2.04 (s, CH 3CO); 13C NMR (125 MHz, CDCl3): δ 154.1 (C-1′), 116.5 (C-2′, C-6′), 128.8 (C-3′, C-5′), 139.0 (C-4′), 28.0 (C-1″), 15.7 (C-2″), 94.3 (C-1), 70.4 (C-2), 70.1 (C-3), 68.3 (C-4), 67.8 (C-5), 61.6 (C-6), 170.5, 170.1, 169.6 (C=O), 20.66, 20.59, 20.57, 20.54 (CH3C=O).
p-Ethylphenyl α-d-Galactopyranoside (3)
High-resolution electron impact mass spectrum (HR-EI-MS) m/z: 284.1258 [M]+, calculated for C14H20O6: 284.1260; [α]D +222.5 (MeOH, c 1.0 g/dl, 20.3°C); IR ν max [cm−1] (neat), 3,366, 1,609, 1,511, 1,228, 1,080, 1,032; 1H NMR (500 MHz, MeOH-d4): δ 6.97 (d, J = 8.9 Hz, H-2′, H-6′), 7.11 (d, J = 8.9 Hz, H-3′, H-5′), 2.58 (q, J = 7.6 Hz, H-1″), 1.19 (t, J = 7.6 Hz, H-2″), 5.43 (d, J = 3.2 Hz, H-1), 3.96–3.91 (overlapping signals, H-2, H-3, H-5), 3.98 (dd, J = 2.8, 1.1 Hz, H-4), 3.71 (dd, J = 11.4, 5.7 Hz, H-6a), 3.67 (dd, J = 11.4, 6.6 Hz, H-6b); 13C NMR (50 MHz, MeOH-d4): δ 156.9 (C-1′), 118.4 (C-2′, C-6′), 129.6 (C-3′, C-5′), 139.5 (C-4′), 29.1 (C-1″), 16.4 (C-2″), 100.0 (C-1), 70.1 (C-2)Footnote 1, 71.4 (C-3)1, 70.8 (C-4), 73.0 (C-5), 62.5 (C-6).
p-Ethylphenyl α-d-Glucopyranoside (4)
HR-FAB-MS m/z: 307.1174 [M + Na]+, calculated for C14H20O6Na: 307.1158; 1H NMR (500 MHz, D2O): δ 6.98 (d, J = 8.7 Hz, H-2′, H-6′), 7.13 (d, J = 8.7 Hz, H-3′, H-5′), 2.47 (q, J = 7.5 Hz, H-1″), 1.04 (t, J = 7.5 Hz, H-2″), 5.46 (d, J = 3.6 Hz, H-1), 3.58 (dd, J = 9.8, 3.6 Hz, H-2), 3.78 (t, J = 9.4 Hz, H-3), 3.38 (t, J = 9.6 Hz, H-4), 3.65 (ddd, J = 9.7, 4.7, 2.5 Hz, H-5), 3.63–3.60 (overlapping signals, H-6a, H-6b); 13C NMR (125 MHz, D2O): δ 154.8 (C-1′), 118.2 (C-2′, C-6′), 129.9 (C-3′, C-5′), 140.5 (C-4′), 28.2 (C-1″), 16.0 (C-2″), 98.2 (C-1), 71.9 (C-2), 73.8 (C-3), 70.2 (C-4), 73.2 (C-5), 61.1 (C-6).
p-Ethylphenyl 2,3,6,2′,3′,4′,6′-Hepta-O-Acetyl-α-Lactoside (5)
HR-EI-MS m/z: 740.2523 [M]+, calculated for C34H44O18: 740.2527; [α]D +83.3 (CHCl3, c 1.0 g/dl, 20.1°C); IR ν max [cm−1] (neat), 1,747, 1,510, 1,369, 1,217, 1,047; 1H NMR (600 MHz, CDCl3): δ 7.00 (d, J = 8.5 Hz, H-2″, H-6″), 7.12 (d, J = 8.5 Hz, H-3″, H-5″), 2.60 (q, J = 7.6 Hz, H-1″′), 1.21 (t, J = 7.6 Hz, H-2″′), 5.60 (d, J = 3.6 Hz, H-1), 4.95 (dd, J = 10.3, 3.7 Hz, H-2), 5.69 (dd, J = 10.2, 9.6 Hz, H-3), 3.84 (t, J = 9.7 Hz, H-4), 4.05 (ddd, J = 10.1, 4.6, 1.9 Hz, H-5), 4.42 (dd, J = 12.0, 1.9 Hz, H-6a), 4.14 (dd, J = 12.1, 4.8 Hz, H-6b), 4.50 (d, J = 7.9 Hz, H-1′), 5.12 (dd, J = 10.3, 7.9 Hz, H-2′), 4.96 (dd, J = 10.4, 3.5 Hz, H-3′), 5.36 (dd, J = 3.6, 1.1 Hz, H-4′), 3.89 (td, J = 7.3, 1.1 Hz, H-5′), 4.16 (dd, J = 11.2, 6.4 Hz, H-6′a), 4.09 (dd, J = 11.2, 7.3 Hz, H-6′b), 2.17, 2.09, 2.08, 2.05, 2.04, 2.02, 1.96 (s, CH 3C=O); 13C NMR (150 MHz, CDCl3): δ 154.2 (C-1″), 116.5 (C-2″, C-6″), 128.8 (C-3″, C-5″), 138.9 (C-4″), 28.0 (C-1″′), 15.8 (C-2″′), 94.3 (C-1), 70.68 (C-2), 69.8 (C-3), 76.2 (C-4), 68.7 (C-5), 61.7 (C-6), 101.0 (C-1′), 69.1 (C-2′), 71.0 (C-3′), 66.6 (C-4′), 70.73 (C-5′), 60.9 (C-6′) 170.32, 170.30, 170.1, 170.0, 169.7, 169.6, 169.1 (C=O), 20.8, 20.7, 20.62, 20.58, 20.57, 20.4 (CH3C=O).
p-Ethylphenyl α-Lactoside (6)
HR-EI-MS m/z: 446.1790 [M]+, calculated for C20H30O11: 446.1788; [α]D +126.3 (MeOH, c 1.0 g/dl, 20.0°C); IR ν max [cm−1] (neat), 3,387, 1,610, 1,510, 1,373, 1,227, 1,070, 1,020; 1H NMR (600 MHz, MeOH-d4): δ 7.05 (d, J = 8.7 Hz, H-2″, H-6″), 7.11 (d, J = 8.8 Hz, H-3″, H-5″), 2.58 (q, J = 7.6 Hz, H-1″′), 1.19 (t, J = 7.6 Hz, H-2″′), 5.42 (d, J = 3.7 Hz, H-1), 3.62 (dd, J = 9.8, 3.7 Hz, H-2), 3.98 (dd, J = 9.7, 8.8 Hz, H-3), 3.67 (dd, J = 9.9, 8.7 Hz, H-4), 3.79 (t, J = 3.3 Hz, H-5), 3.87 (dd, J = 12.2, 3.6 Hz, H-6a), 3.74 (dd, J = 12.3, 3.5 Hz, H-6b), 4.38 (d, J = 7.7 Hz, H-1′), 3.56 (dd, J = 9.7, 7.7 Hz, H-2′), 3.49 (dd, J = 9.8, 3.3 Hz, H-3′), 3.82 (dd, J = 3.3, 0.7 Hz, H-4′), 3.60 (ddd, J = 7.5, 4.7, 0.9 Hz, H-5′), 3.80 (dd, J = 11.4, 7.5 Hz, H-6′a), 3.71 (dd, J = 11.5, 4.6 Hz, H-6′b); 13C NMR (150 MHz, MeOH-d4): δ 156.6 (C-1″), 118.1 (C-2″, C-6″), 129.7 (C-3″, C-5″), 139.6 (C-4″), 29.1 (C-1″′), 16.5 (C-2″′), 99.2 (C-1), 73.1 (C-2), 73.4 (C-3), 80.6 (C-4), 77.2 (C-5), 62.6 (C-6), 105.2 (C-1′), 72.7 (C-2′)1, 74.9 (C-3′), 70.4 (C-4′), 72.6 (C-5′)1, 61.6 (C-6′).
p-(1″-Hydroxyethyl)-Phenyl 2,3,4,6-Tetra-O-Acetyl-α-d-Galactopyranoside (Mixture of Epimers 7)
HR-FAB-MS m/z: 491.1509 [M + Na]+, calculated for C22H28O11Na: 491.1529; IR ν max [cm−1] (neat), 3,481, 1,745, 1,608, 1,510, 1,371, 1,217, 1,066; 1H NMR (500 MHz, CDCl3): δ 7.04 (d, J = 8.6 Hz, H-2′, H-6′), 7.32 (d, J = 8.6 Hz, H-3′, H-5′), 4.87 (q, J = 6.4 Hz, H-1″), 1.48/1.47 (d, J = 6.5 Hz, H-2″)Footnote 2, 5.76 (d, J = 3.2 Hz, H-1), 5.28 (dd, J = 10.9, 3.6 Hz, H-2), 5.58 (dd, J = 10.9, 3.4 Hz, H-3), 5.53 (dd, J = 3.4, 1.0 Hz, H-4), 4.35 (t, J = 6.6 Hz, H-5), 4.12 (dd, J = 11.3, 6.3 Hz, H-6a), 4.07 (dd, J = 11.3, 6.6 Hz, H-6b)/4.07 (dd, J = 11.2, 6.6 Hz, H-6b)2, 2.17, 2.08, 2.03, 1.95 (s, CH 3CO); 13C NMR (50 MHz, CDCl3): δ 155.6 (C-1′), 116.7 (C-2′, C-6′), 126.7 (C-3′, C-5′), 140.5 (C-4′), 69.8/69.7 (C-1″)2, 25.18/25.16 (C-2″)2, 95.0 (C-1), 67.78 (C-2), 67.5 (C-3), 67.83 (C-4), 67.1 (C-5), 61.4 (C-6), 170.4, 170.3, 170.2, 170.0 (C=O), 20.7, 20.63, 20.59, 20.55 (CH3C=O).
p-(1″-Hydroxyethyl)-Phenyl 2,3,4,6-Tetra-O-Acetyl-α-d-Glucopyranoside (Mixture of Epimers 8)
HR-FAB-MS m/z: 491.1509 [M + Na]+, calculated for C22H28O11Na: 491.1529; IR ν max [cm−1] (neat), 3,500, 1,751, 1,608, 1,508, 1,369, 1,219, 1,043; 1H NMR (500 MHz, CDCl3): δ 7.07 (d, J = 8.6 Hz, H-2′, H-6′), 7.32 (d, J = 8.6 Hz, H-3′, H-5′), 4.88 (q, J = 6.4 Hz, H-1″), 1.48/1.48 (d, J = 6.5 Hz, H-2″)Footnote 3, 5.73 (d, J = 3.8 Hz, H-1)/5.73 (d, J = 4.0 Hz, H-1)3, 5.04 (dd, J = 10.2, 3.7 Hz, H-2), 5.71 (t, J = 9.9 Hz, H-3), 5.16 (dd, J = 10.2, 9.5 Hz, H-4), 4.12 (ddd, J = 10.3, 4.4, 2.2 Hz, H-5), 4.25 (dd, J = 12.3, 4.5 Hz, H-6a), 4.05/4.04 (dd, J = 12.3, 1.9 Hz, H-6b)3, 2.06, 2.05, 2.04, 2.04 (s, CH 3CO); 13C NMR (125 MHz, CDCl3): δ 155.3 (C-1′), 116.5 (C-2′, C-6′), 126.7 (C-3′, C-5′), 140.6 (C-4′), 69.7/69.6 (C-1″)3, 25.2 (C-2″), 94.3 (C-1), 70.4 (C-2), 70.0 (C-3), 68.2 (C-4), 67.9 (C-5), 61.5 (C-6), 170.5, 170.14, 170.12, 169.6 (C=O), 20.7, 20.61, 20.57, 20.55 (CH3C=O).
p-(1″-Hydroxyethyl)-Phenyl α-d-Galactopyranoside (Mixture of Epimers 9)
HR-EI-MS m/z: 300.1210 [M]+, calculated for C14H20O7: 300.1209; 1H NMR (500 MHz, MeOH-d4): δ 7.12 (d, J = 8.5 Hz, H-2′, H-6′), 7.28 (d, J = 8.5 Hz, H-3′, H-5′), 4.77 (q, J = 6.5 Hz, H-1″), 1.40 (d, J = 6.5 Hz, H-2″), 5.47 (d, J = 3.0 Hz, H-1)/5.47 (d, J = 2.5 Hz, H-1)3, 3.96–3.93 (overlapping signals, H-2, H-3), 3.97 (dd, J = 2.5, 1.3 Hz, H-4), 3.92 (t, J = 6.3 Hz, H-5), 3.69 (dd, J = 11.3, 5.7 Hz, H-6a), 3.66/3.66 (dd, J = 11.4, 6.7 Hz, H-6b)3; 13C NMR (125 MHz, MeOH-d4): δ 157.9 (C-1′), 118.2 (C-2′, C-6′), 127.6 (C-3′, C-5′), 141.5 (C-4′), 70.44/70.40 (C-1″)3, 25.5 (C-2″), 99.8 (C-1), 70.0 (C-2)Footnote 4, 71.4 (C-3)4, 70.8 (C-4), 73.0 (C-5), 62.4 (C-6).
p-(1″-Hydroxyethyl)-Phenyl α-d-Glucopyranoside (Mixture of Epimers 10)
HR-EI-MS m/z: 300.1212 [M]+, calculated for C14H20O7: 300.1209; 1H NMR (500 MHz, MeOH-d4): δ 7.12 (d, J = 8.5 Hz, H-2′, H-6′), 7.28 (d, J = 8.6 Hz, H-3′, H-5′), 4.77 (q, J = 6.4 Hz, H-1″), 1.40 (d, J = 6.5 Hz, H-2″), 5.46 (d, J = 3.6 Hz, H-1), 3.56 (dd, J = 9.7, 3.7 Hz, H-2), 3.85 (t, J = 9.3 Hz, H-3), 3.43 (t, J = 9.3 Hz, H-4), 3.65 (ddd, J = 9.6, 4.6, 2.5 Hz, H-5), 3.72 (dd, J = 11.7, 2.1 Hz, H-6a), 3.68 (dd, J = 11.8, 4.6 Hz, H-6b); 13C NMR (125 MHz, MeOH-d4): δ 157.7 (C-1′), 118.0 (C-2′, C-6′), 127.7 (C-3′, C-5′), 141.5 (C-4′), 70.41/70.37 (C-1″)3, 25.5 (C-2″), 99.4 (C-1), 73.3 (C-2), 74.9 (C-3), 71.5 (C-4), 74.3 (C-5), 62.3 (C-6).
p-(1″-Acetoxyethyl)-Phenyl 2,3,4,6-Tetra-O-Acetyl-α-d-Galactopyranoside (Mixture of Epimers 11)
HR-CI-MS m/z: 510.1757 [M]+, calculated for C24H30O12: 510.1737; 1H NMR (500 MHz, CDCl3): δ 7.04 (d, J = 8.5 Hz, H-2′, H-6′)/7.03 (d, J = 8.7 Hz, H-2′, H-6′)3, 7.30 (d, J = 8.5 Hz, H-3′, H-5′), 5.84/5.84 (q, J = 6.5 Hz, H-1″)3, 1.52/1.51 (d, J = 6.5 Hz, H-2″)3, 5.77/5.76 (d, J = 3.6 Hz, H-1)3, 5.28 (dd, J = 10.9, 3.7 Hz, H-2)/5.27 (dd, J = 11.0, 3.6 Hz, H-2)3, 5.57 (dd, J = 10.9, 3.5 Hz, H-3), 5.52 (d, J = 3.4 Hz, H-4), 4.33 (t, J = 6.8 Hz, H-5), 4.12 (dd, J = 11.2, 6.5 Hz, H-6a), 4.07 (dd, J = 11.2, 6.9 Hz, H-6b), 2.17, 2.06, 2.05, 2.05, 2.03 (s, CH 3CO); 13C NMR (50 MHz, CDCl3): δ 155.8 (C-1′), 116.6 (C-2′, C-6′), 127.6 (C-3′, C-5′), 136.3 (C-4′), 71.74/71.69 (C-1″)3, 22.0 (C-2″), 94.7 (C-1), 67.7 (C-2), 67.4 (C-3), 67.8 (C-4), 67.1 (C-5), 61.3 (C-6), 170.3, 170.2, 170.1, 170.0 (C=O), 21.3, 20.59, 20.55, 20.47 (CH3C=O).
p-(1″-Acetoxyethyl)-Phenyl 2,3,4,6-Tetra-O-Acetyl-α-d-Glucopyranoside (Mixture of Epimers 12)
HR-CI-MS m/z: 510.1708 [M]+, calculated for C24H30O12: 510.1737; 1H NMR (500 MHz, CDCl3): δ 7.07/7.06 (d, J = 8.8 Hz, H-2′, H-6′)3, 7.30 (d, J = 8.8 Hz, H-3′, H-5′), 5.84/5.83 (q, J = 6.5 Hz, H-1″)3, 1.51 (d, J = 6.7 Hz, H-2″), 5.73/5.72 (d, J = 3.7 Hz, H-1)3, 5.04 (dd, J = 10.3, 3.6 Hz, H-2)/5.03 (dd, J = 10.2, 3.7 Hz, H-2)3, 5.70 (t, J = 9.9 Hz, H-3), 5.16 (dd, J = 10.1, 9.4 Hz, H-4)/5.15 (dd, J = 10.2, 9.5 Hz, H-4)3, 4.10 (ddd, J = 10.2, 4.3, 2.1 Hz, H-5), 4.25/4.24 (dd, J = 12.3, 4.3 Hz, H-6a)3, 4.05 (dd, J = 12.4, 2.0 Hz, H-6b), 2.17, 2.06, 2.04 (s, CH 3CO); 13C NMR (125 MHz, CDCl3): δ 155.7/155.6 (C-1′)3, 116.5 (C-2′, C-6′), 127.6 (C-3′, C-5′), 136.39/136.37 (C-4′)3, 71.8/71.7 (C-1″)3, 22.04/22.02 (C-2″)3, 94.20/94.15 (C-1)3, 70.4 (C-2), 70.0 (C-3), 68.3 (C-4), 68.0 (C-5), 61.5 (C-6), 170.5, 170.2, 170.11, 170.08, 169.5 (C=O), 21.3, 20.65, 20.58, 20.55 (CH3C=O).
p-Vinylphenyl 2,3,4,6-Tetra-O-Acetyl-α-d-Galactopyranoside (13)
HR-EI-MS m/z: 450.1522 [M]+, calculated for C22H26O10: 450.1526; 1H NMR (500 MHz, CDCl3): δ 7.02 (d, J = 8.7 Hz, H-2′, H-6′), 7.35 (d, J = 8.7 Hz, H-3′, H-5′), 6.66 (dd, J = 17.8, 10.9 Hz, H-1″), 5.65 (dd, J = 17.6, 0.7 Hz, H-2″trans), 5.18 (dd, J = 10.9, 0.5 Hz, H-2″cis), 5.78 (d, J = 3.7 Hz, H-1), 5.29 (dd, J = 10.7, 3.7 Hz, H-2), 5.58 (dd, J = 10.8, 3.3 Hz, H-3), 5.53 (dd, J = 3.3, 1.0 Hz, H-4), 4.34 (td, J = 7.0, 0.9 Hz, H-5), 4.12 (dd, J = 11.3, 6.3 Hz, H-6a), 4.06 (dd, J = 11.3, 7.1 Hz, H-6b), 2.17, 2.08, 2.03, 1.94 (s, CH 3C=O); 13C NMR (125 MHz, CDCl3): δ 155.9 (C-1′), 116.8 (C-2′, C-6′), 127.4 (C-3′, C-5′), 132.7 (C-4′), 135.9 (C-1″), 112.9 (C-2″), 94.8 (C-1), 67.8 (C-2), 67.5 (C-3), 67.9 (C-4), 67.2 (C-5), 61.4 (C-6), 170.4, 170.3, 170.2, 170.0 (C=O), 20.7, 20.65, 20.61, 20.5 (CH3C=O).
p-Vinylphenyl 2,3,4,6-Tetra-O-Acetyl-α-d-Glucopyranoside (14)
HR-EI-MS m/z: 450.1534 [M]+, calculated for C22H26O10: 450.1526; 1H NMR (500 MHz, CDCl3): δ 7.05 (d, J = 8.7 Hz, H-2′, H-6′), 7.36 (d, J = 8.7 Hz, H-3′, H-5′), 6.66 (dd, J = 17.5, 10.9 Hz, H-1″), 5.65 (dd, J = 17.5, 0.7 Hz, H-2″trans), 5.19 (dd, J = 10.8, 0.7 Hz, H-2″cis), 5.74 (d, J = 3.7 Hz, H-1), 5.04 (dd, J = 10.3, 3.6 Hz, H-2), 5.70 (t, J = 9.9 Hz, H-3), 5.16 (t, J = 9.4 Hz, H-4), 4.11 (ddd, J = 10.3, 4.4, 2.2 Hz, H-5), 4.25 (dd, J = 12.3, 4.6 Hz, H-6a), 4.05 (dd, J = 12.3, 2.3 Hz, H-6b), 2.06, 2.05, 2.04, 2.04 (s, CH 3CO); 13C NMR (125 MHz, CDCl3): δ 155.7 (C-1′), 116.6 (C-2′, C-6′), 127.4 (C-3′, C-5′), 132.7 (C-4′), 135.8 (C-1″), 112.9 (C-2″), 94.2 (C-1), 70.4 (C-2), 70.0 (C-3), 68.3 (C-4), 68.0 (C-5), 61.6 (C-6), 170.5, 170.1, 169.6 (C=O), 20.7, 20.62, 20.60, 20.57 (CH3C=O).
p-Vinylphenyl α-d-Galactopyranoside (15)
HR-EI-MS m/z: 282.1097 [M]+, calculated for C14H18O6: 282.1103; [α]D +275.8 (MeOH, c 1.0 g/dl, 20.1°C); IR ν max [cm−1] (neat), 3,389, 1,629, 1,605, 1,508, 1,234, 1,082, 1,033; 1H NMR (500 MHz, MeOH-d4): δ 7.12 (d, J = 8.8 Hz, H-2′, H-6′), 7.35 (d, J = 8.8 Hz, H-3′, H-5′), 6.66 (dd, J = 17.7, 11.0 Hz, H-1″), 5.63 (dd, J = 17.5, 0.9 Hz, H-2″trans), 5.10 (dd, J = 11.0, 0.9 Hz, H-2″cis), 5.49 (d, J = 2.2 Hz, H-1), 3.89–3.84 (overlapping signals, H-2, H-3), 3.97 (dd, J = 1.8, 1.1 Hz, H-4), 3.91 (t, J = 6.3 Hz, H-5), 3.70 (dd, J = 11.4, 5.6 Hz, H-6a), 3.66 (dd, J = 11.4, 6.6 Hz, H-6b); 13C NMR (125MHz, MeOH-d4): δ 158.5 (C-1′), 118.3 (C-2′, C-6′), 128.3 (C-3′, C-5′), 133.4 (C-4′), 137.5 (C-1″), 112.4 (C-2″), 99.7 (C-1), 70.0 (C-2)Footnote 5, 71.4 (C-3)5, 70.8 (C-4), 73.1 (C-5), 62.4 (C-6).
p-Vinylphenyl α-d-Glucopyranoside (16)
HR-EI-MS m/z: 282.1102 [M]+, calculated for C14H18O6: 282.1103; 1H NMR (500 MHz, MeOH-d4): δ 7.11 (d, J = 8.8 Hz, H-2′, H-6′), 7.35 (d, J = 8.5 Hz, H-3′, H-5′), 6.66 (dd, J = 17.7, 11.0 Hz, H-1″), 5.64 (dd, J = 17.7, 0.8 Hz, H-2″trans), 5.11 (dd, J = 11.0, 0.7 Hz, H-2″cis), 5.47 (d, J = 3.6 Hz, H-1), 3.56 (dd, J = 9.7, 3.7 Hz, H-2), 3.84 (t, J = 9.3 Hz, H-3), 3.42 (t, J = 9.4 Hz, H-4), 3.64 (ddd, J = 9.8, 4.7, 2.5 Hz, H-5), 3.73 (dd, J = 11.9, 2.5 Hz, H-6a), 3.68 (dd, J = 11.9, 4.7 Hz, H-6b); 13C NMR (50 MHz, MeOH-d4): δ 158.3 (C-1′), 118.1 (C-2′, C-6′), 128.3 (C-3′, C-5′), 133.4 (C-4′), 137.5 (C-1″), 112.4 (C-2″), 99.2 (C-1), 73.3 (C-2), 74.9 (C-3), 71.5 (C-4), 74.4 (C-5), 62.3 (C-6).
p -Ethylphenyl 2,3,4,6-Tetra- O -Acetyl-β- d -Glucopyranoside Footnote 6 ( 17 )
HR-EI-MS m/z: 452.1654 [M]+, calculated for C22H28O10: 452.1682; [α]D −22.2 (CHCl3, c 1.0 g/dl, 20.7°C); IR ν max [cm−1] (neat), 1,751, 1,608, 1,510, 1,367, 1,221, 1,047; 1H NMR (600 MHz, CDCl3): δ 6.92 (d, J = 8.7 Hz, H-2′, H-6′), 7.12 (d, J = 8.7 Hz, H-3′, H-5′), 2.60 (q, J = 7.6 Hz, H-1″), 1.21 (t, J = 7.6 Hz, H-2″), 5.05 (d, J = 7.6 Hz, H-1), 5.26 (dd, J = 9.2, 7.5 Hz, H-2), 5.29 (t, J = 9.2 Hz, H-3), 5.17 (t, J = 9.5 Hz, H-4), 3.85 (ddd, J = 10.0, 5.3, 2.5 Hz, H-5), 4.29 (dd, J = 12.3, 5.3 Hz, H-6a), 4.17 (dd, J = 12.3, 2.4 Hz, H-6b), 2.08, 2.06, 2.05, 2.04 (s, CH 3C=O); 13C NMR (150 MHz, CDCl3): δ 154.9 (C-1′), 116.9 (C-2′, C-6′), 128.7 (C-3′, C-5′), 139.2 (C-4′), 28.0 (C-1″), 15.7 (C-2″), 99.3 (C-1), 71.1 (C-2), 72.7 (C-3), 68.2 (C-4), 71.8 (C-5), 61.9 (C-6), 170.5, 170.2, 169.3, 169.2 (C=O), 20.61, 20.55, 20.53, 20.50 (CH3C=O).
p-Ethylphenyl β-d-Glucopyranoside (18)
HR-FAB-MS m/z: 307.1156 [M + Na]+, calculated for C14H20O6Na: 307.1158; [α]D −65.4 (MeOH, c 1.0 g/dl, 19.9°C); IR ν max [cm−1] (neat), 3,335, 1,611, 1,511, 1,233, 1,070; 1H NMR (500 MHz, D2O): δ 6.96 (d, J = 8.5 Hz, H-2′, H-6′), 7.14 (d, J = 8.5 Hz, H-3′, H-5′), 2.49 (q, J = 7.6 Hz, H-1″), 1.06 (t, J = 7.5 Hz, H-2″), 4.96 (d, J = 7.6 Hz, H-1), 3.43 (dd, J = 9.4, 7.6 Hz, H-2), 3.48 (t, J = 9.1 Hz, H-3), 3.36 (t, J = 9.3 Hz, H-4), 3.48 (ddd, J = 9.9, 5.7, 2.0 Hz, H-5), 3.80 (dd, J = 12.3, 1.8 Hz, H-6a), 3.62 (dd, J = 12.4, 5.7 Hz, H-6b); 13C NMR (50 MHz, MeOH-d4): δ 157.1 (C-1′), 117.7 (C-2′, C-6′), 129.6 (C-3′, C-5′), 139.4 (C-4′), 29.0 (C-1″), 16.4 (C-2″), 102.5 (C-1), 74.8 (C-2), 77.9 (C-3)5, 71.3 (C-4), 78.0 (C-5)5, 62.4 (C-6).
p-Ethylphenyl 2,3,6,2′,3′,4′,6′-Hepta-O-Acetyl-β-Lactoside (19)
HR-FAB-MS m/z: 763.2396 [M + Na]+, calculated for C34H44O18Na: 763.2425; [α]D −22.4 (CHCl3, c 1.0 g/dl, 22.4°C); IR ν max [cm−1] (neat), 3,028, 1,751, 1,608, 1,508, 1,369, 1,219, 1,057; 1H NMR (600 MHz, CDCl3): δ 6.89 (d, J = 8.6 Hz, H-2″, H-6″), 7.10 (d, J = 8.7 Hz, H-3″, H-5″), 2.60 (q, J = 7.6 Hz, H-1″′), 1.20 (t, J = 7.6 Hz, H-2″′), 5.01 (d, J = 7.8 Hz, H-1), 5.16 (dd, J = 9.3, 7.8 Hz, H-2), 5.27 (t, J = 9.1 Hz, H-3), 3.89 (t, J = 9.4 Hz, H-4), 3.77 (ddd, J = 9.9, 5.8, 2.2 Hz, H-5), 4.50 (dd, J = 12.3, 2.6 Hz, H-6a), 4.15 (dd, J = 12.0, 5.6 Hz, H-6b), 4.51 (d, J = 8.0 Hz, H-1′), 5.13 (dd, J = 10.4, 7.9 Hz, H-2′), 4.97 (dd, J = 10.4, 3.5 Hz, H-3′), 5.36 (dd, J = 3.4, 1.0 Hz, H-4′), 3.90 (t, J = 7.1 Hz, H-5′), 4.13 (dd, J = 11.0, 6.8 Hz, H-6′a), 4.10 (dd, J = 11.2, 7.3 Hz, H-6′b), 2.16, 2.09, 2.08, 2.07, 2.06, 1.97 (s, CH 3C=O); 13C NMR (150 MHz, CDCl3): δ 154.8 (C-1″), 116.8 (C-2″, C-6″), 128.7 (C-3″, C-5″), 139.2 (C-4″), 28.0 (C-1″′), 15.7 (C-2″′), 99.0 (C-1), 71.5 (C-2), 72.8 (C-3), 76.2 (C-4), 72.7 (C-5), 62.0 (C-6), 101.0 (C-1′), 69.0 (C-2′), 70.9 (C-3′), 66.6 (C-4′), 70.7 (C-5′), 60.8 (C-6′) 170.41, 170.38, 170.3, 170.2, 170.1, 169.6, 169.0 (C= O), 20.9, 20.8, 20.67, 20.65, 20.62, 20.59, 20.50 (CH3C=O).
p-Ethylphenyl β-Lactoside (20)
1H NMR (300 MHz, pyridin-d 5 ): δ 7.26 (d, J = 8.7 Hz, H-2″, H-6″), 7.43 (d, J = 8.7 Hz, H-3″, H-5″), 2.64 (q, J = 6.8 Hz, H-1″′), 1.26 (t, J = 6.8 Hz, H-2″′), 5.73 (d, J = 7.5 Hz, H-1), 4.81–4.20 (overlapping signals H-2, H-3, H-4, H-6a, H-6b, H-2′, H-3′, H-4′, H-5′, H-6′a, H-6′b), 4.24 (ddd, J = 9.3, 6.3, 2.7 Hz, H-5), 5.31 (d, J = 8.1 Hz, H-1′); 13C NMR (75 MHz, pyridin-d 5 ): δ 156.7 (C-1″), 117.1 (C-2″, C-6″), 129.2 (C-3″, C-5″), 138.0 (C-4″), 28.3 (C-1″′), 16.2 (C-2″′), 102.0 (C-1), 74.6 (C-2), 76.8 (C-3), 81.8 (C-4), 76.8 (C-5), 61.9 (C-6), 105.9 (C-1′), 70.2 (C-2′), 72.6 (C-3′), 77.4 (C-4′), 75.3 (C-5′), 62.1 (C-6′).
p -(1″-Hydroxyethyl)-Phenyl 2,3,4,6-Tetra- O -Acetyl-β- d -Glucopyranoside Footnote 7 (Mixture of Epimers 21 )
HR-CI-MS m/z: 469.1716 [M + H]+, calculated for C22H29O11: 469.1710; 1H NMR (500 MHz, CDCl3): δ 6.97 (d, J = 8.6 Hz, H-2′, H-6′), 7.31 (d, J = 8.6 Hz, H-3′, H-5′), 4.88 (q, J = 5.8 Hz, H-1″), 1.48 (d, J = 6.5 Hz, H-2″), 5.07 (d, J = 7.6 Hz, H-1)/5.07 (d, J = 7.4 Hz, H-1)Footnote 8, 5.27 (t, J = 9.1 Hz, H-2), 5.30 (t, J = 9.0 Hz, H-3), 5.17 (t, J = 9.4 Hz, H-4), 3.86 (ddd, J = 9.9, 5.3, 2.3 Hz, H-5), 4.29 (dd, J = 12.3, 5.2 Hz, H-6a), 4.17 (dd, J = 12.3, 2.2 Hz, H-6b)/4.17 (dd, J = 12.2, 2.2 Hz, H-6b)8, 2.08, 2.06, 2.05, 2.04 (s, CH 3CO); 13C NMR (125 MHz, CDCl3): δ 156.2 (C-1′), 117.06/117.04 (C-2′, C-6′)8, 126.7 (C-3′, C-5′), 140.9 (C-4′), 69.9/69.8 (C-1″)8, 25.23/25.22 (C-2″)8, 99.2 (C-1), 71.2 (C-2), 72.7 (C-3), 68.3 (C-4), 72.1 (C-5), 62.0 (C-6), 170.5, 170.2, 169.4, 169.3 (C=O), 20.7, 20.64, 20.63, 20.60 (CH3C=O).
p-(1″-Hydroxyethyl)-Phenyl β-d-Glucopyranoside (Mixture of Epimers 22)
HR-EI-MS m/z: 300.1198 [M]+, calculated for C14H20O7: 300.1209; IR ν max [cm−1] (neat), 3,367, 1,610, 1,510, 1,230, 1,068; 1H NMR (500 MHz, D2O): δ 7.00 (d, J = 8.8 Hz, H-2′, H-6′), 7.26 (d, J = 8.8 Hz, H-3′, H-5′), 4.75 (q, J = 6.5 Hz, H-1″), 1.32 (d, J = 6.5 Hz, H-2″), 4.98 (d, J = 7.5 Hz, H-1), 3.43 (dd, J = 8.9, 7.5 Hz, H-2), 3.48 (t, J = 8.9 Hz, H-3), 3.36 (dd, J = 9.8, 8.9 Hz, H-4), 3.48 (ddd, J = 9.8, 5.5, 2.4 Hz, H-5), 3.79 (dd, J = 12.4, 2.1 Hz, H-6a), 3.62 (dd, J = 12.4, 5.7 Hz, H-6b); 13C NMR (125 MHz, D2O): δ 156.7 (C-1′), 117.5 (C-2′, C-6′), 128.0 (C-3′, C-5′), 140.6 (C-4′), 70.10/70.06 (C-1″)8, 24.3 (C-2″), 101.1 (C-1), 73.8 (C-2), 76.9 (C-3), 70.3 (C-4), 76.4 (C-5), 61.4 (C-6).
p-(1″-Acetoxyethyl)-Phenyl 2,3,4,6-Tetra-O-Acetyl-β-d-Glucopyranoside (Mixture of Epimers 23)
HR-EI-MS m/z: 510.1736 [M]+, calculated for C24H30O12: 510.1737; 1H NMR (500 MHz, CDCl3): δ 6.99 (d, J = 8.7 Hz, H-2′, H-6′), 7.31 (d, J = 8.6 Hz, H-3′, H-5′), 5.86 (q, J = 6.5 Hz, H-1″), 1.51 (d, J = 6.5 Hz, H-2″), 5.08/5.07 (d, J = 7.6 Hz, H-1)8, 5.29 (dd, J = 9.3, 7.5 Hz, H-2), 5.32 (t, J = 9.1 Hz, H-3), 5.19 (t, J = 9.5 Hz, H-4), 3.87 (ddd, J = 10.0, 5.3, 2.5 Hz, H-5), 4.31 (dd, J = 12.3, 5.3 Hz, H-6a), 4.18/4.17 (dd, J = 12.3, 2.3 Hz, H-6b)8, 2.08/2.088, 2.06, 2.05, 2.05, 2.04 (s, CH 3CO); 13C NMR (125 MHz, CDCl3): δ 156.4 (C-1′), 116.9 (C-2′, C-6′), 127.57/127.55 (C-3′, C-5′)8, 136.7 (C-4′), 71.8 (C-1″), 22.1/22.0 (C-2″)8, 99.0 (C-1), 71.1 (C-2), 72.7 (C-3), 68.3 (C-4), 72.0 (C-5), 61.9 (C-6), 170.6, 170.3, 170.2, 169.4, 169.3 (C=O), 21.3, 20.7, 20.62, 20.60, 20.58 (CH3C=O).
p-Vinylphenyl 2,3,4,6-Tetra-O-Acetyl-β-d-Glucopyranoside7 (24)
HR-EI-MS m/z: 450.1518 [M]+, calculated for C22H26O10: 450.1526; [α]D −20.8 (CHCl3, c 1.0 g/dl, 21.2°C); IR ν max [cm−1] (neat), 3,022, 1,755, 1,630, 1,606, 1,508, 1,367, 1,221, 1,047; 1H NMR (500 MHz, CDCl3): δ 6.95 (d, J = 8.7 Hz, H-2′, H-6′), 7.34 (d, J = 8.7 Hz, H-3′, H-5′), 6.66 (dd, J = 17.8, 10.9 Hz, H-1″), 5.65 (dd, J = 17.5, 0.7 Hz, H-2″trans), 5.19 (dd, J = 10.9, 0.7 Hz, H-2″cis), 5.08 (d, J = 7.5 Hz, H-1), 5.27 (dd, J = 9.1, 7.5 Hz, H-2), 5.30 (t, J = 9.0 Hz, H-3), 5.17 (t, J = 9.5 Hz, H-4), 3.86 (ddd, J = 10.0, 5.2, 2.5 Hz, H-5), 4.29 (dd, J = 12.3, 5.5 Hz, H-6a), 4.17 (dd, J = 12.3, 2.5 Hz, H-6b), 2.08, 2.06, 2.05, 2.04 (s, CH 3CO); 13C NMR (125 MHz, CDCl3): δ 156.4 (C-1′), 117.0 (C-2′, C-6′), 127.3 (C-3′, C-5′), 133.0 (C-4′), 135.8 (C-1″), 113.0 (C-2″), 99.1 (C-1), 71.1 (C-2), 72.7 (C-3), 68.3 (C-4), 72.0 (C-5), 61.9 (C-6), 170.5, 170.2, 169.3, 169.2 (C=O), 20.63, 20.58, 20.56, 20.54 (CH3C=O).
p-Vinylphenyl β-d-Glucopyranoside7 (25)
HR-EI-MS m/z: 282.1097 [M]+, calculated for C14H18O6: 282.1103; [α]D −81.5 (MeOH, c 1.0 g/dl, 21.0°C); IR ν max [cm−1] (neat), 3,318, 1,627, 1,606, 1,509, 1,245, 1,076, 1,046; 1H NMR (600 MHz, MeOH-d4): δ 7.04 (d, J = 8.5 Hz, H-2′, H-6′), 7.38 (d, J = 8.5 Hz, H-3′, H-5′), 6.66 (dd, J = 17.6, 10.9 Hz, H-1″), 5.64 (dd, J = 17.3, 0.9 Hz, H-2″trans), 5.11 (dd, J = 11.0, 0.9 Hz, H-2″cis), 4.90 (d, J = 7.6 Hz, H-1), 3.46–3.36 (overlapping signals, H-2, H-3, H-4, H-5), 3.89 (dd, J = 12.1, 2.0 Hz, H-6a), 3.68 (dd, J = 12.1, 5.6 Hz, H-6b); 13C NMR (150 MHz, MeOH-d4): δ 158.8 (C-1′), 117.7 (C-2′, C-6′), 128.3 (C-3′, C-5′), 133.5 (C-4′), 137.5 (C-1″), 112.4 (C-2″), 102.2 (C-1), 74.9 (C-2), 78.2 (C-3), 71.4 (C-4), 78.0 (C-5), 62.5 (C-6).
Rights and permissions
About this article
Cite this article
Socolsky, C., Fascio, M.L., D’Accorso, N.B. et al. Effects of p-Vinylphenyl Glycosides and Other Related Compounds on the Oviposition Behavior of Ceratitis capitata . J Chem Ecol 34, 539–548 (2008). https://doi.org/10.1007/s10886-008-9440-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-008-9440-4