Abstract
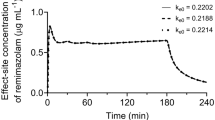
This study is the first to report 50% and 95% effect-site concentrations (EC50 and EC95, respectively) of the new short-acting benzodiazepine, remimazolam, for the successful insertion of i-gels with co-administration of fentanyl. Thirty patients (38 ± 5 years old, male/female = 4/26) were randomly assigned into five groups to receive one of five different remimazolam doses (0.1, 0.15, 0.2, 0.25, and 0.3 mg/kg bolus followed by infusion of 1, 1.5, 2, 2.5, and 3 mg/kg/h, respectively, for 10 min), which were designed to maintain a constant effect-site concentration of remimazolam at the time of i-gel insertion. At 6 min after the start of remimazolam infusion, all patients received 2 µg/kg fentanyl. i-gel insertion was attempted at 10 min and the success or failure of insertion were assessed by the patient response. Probit analysis was used to estimate the EC50 and EC95 values of remimazolam with 95% confidence intervals (CIs). In the five remimazolam dose groups, two, two, four, five, and six of the six patients in each group had an i-gel successfully inserted. Two patients in the lowest remimazolam dose group were conscious at the time of i-gel insertion and were counted as failures. The EC50 and EC95 values of remimazolam were 0.88 (95% CI, 0.65–1.11) and 1.57 (95% CI, 1.09–2.05) µg/ml, respectively. An effect-site concentration of ≥ 1.57 µg/ml was needed to insert an i-gel using remimazolam anesthesia, even with 2 µg/kg fentanyl.
Trial registration: The study was registered in Japan Registry of Clinical Trials on 19 April 2021, Code jRCTs041210009.



Similar content being viewed by others
References
Lohmer LL, Schippers F, Petersen KU, Stoehr T, Schmith VD. Time-to-event modeling for Remimazolam for the indication of induction and maintenance of General Anesthesia. J Clin Pharmacol. 2020;60:505–14. https://doi.org/10.1002/jcph.1552.
Pesic M, Schippers F, Saunders R, Webster L, Donsbach M, Stoehr T. Pharmacokinetics and pharmacodynamics of intranasal remimazolam -a randomized controlled clinical trial. Eur J Clin Pharmacol. 2020;76:1505–16. https://doi.org/10.1007/s00228-020-02984-z.
Kilpatrick GJ, McIntyre MS, Cox RF, Stafford JA, Pacofsky GJ, Lovell GG, Wiard RP, Feldman PL, Collins H, Waszczak BL, Tilbook GS. CNS 7056: a novel ultra-short-acting benzodiazepine. Anesthesiology. 2007;107:60–6. https://doi.org/10.1097/01.anes.0000267503.85085.c0.
Gibbison B, Cook TM, Seller C. Case series: protection from aspiration and failure of protection from aspiration with i-gel airway. Br J Anaesth. 2008;100:415–7. https://doi.org/10.1093/bja/aem396.
Levitan RM, Kinkle WC. Initial anatomic investigations of the I-gel airway: a novel supraglottic airway without inflatable cuff. Anaesthesia. 2005;60:1022–6. https://doi.org/10.1111/j.1365-2044.2005.04258.x.
Thomson IR. The haemodynamic response to intubation:a perspective. Can J Anaesth. 1989;36:367–9. https://doi.org/10.1007/BF03005331.
Ismail SA, Bishre NA, Kandil HW, Mowafi HA, Atawia HA. Intraocular pressure and haemodynamic responses to insertion of the i-gel, laryngeal mask airway or endotracheal tube. Eur J Anaesthesiol. 2011;28:443–8.
Elgebaly AS, Eldabaa AA. Is I-gel airway a better option to endotracheal tube airway for sevoflurane-fentanyl anesthesia during cardiac surgery? Anesth Essays Res. 2014;8:216–22.
O’Meara ME, Jones JG. The laryngeal mask. BMJ. 1993;306:224–5. https://doi.org/10.1136/bmj.306.6872.224.
Brimacombe J, Berry A. The laryngeal mask airway-anatomical and physiological implications. Acta Anaesthesiol Scand. 1996;40:201–9. https://doi.org/10.1111/j.1399-6576.1996.tb04420.x.
Kim J, Lee S, Kim Y, Jeong JS. Remimazolam dose for successful insertion of a supraglottic airway device with opioids: a dose-determination study using Dixon’s up-and-down method. Can J Anaesth. 2023;70:343–50. https://doi.org/10.1007/s12630-022-02379-x.
Oh J, Park SY, Lee GY, Park JH, Joe HB. Effective dose of remimazolam co-administered with remifentanil to facilitate I-gel insertion without neuromuscular blocking agents: an up-and-down sequential allocation trial. BMC Anesthesiol. 2023;23:81. https://doi.org/10.1186/s12871-023-02041-z.
Muzi M, Robinson BJ, Ebert TJ, O’Brien TJ. Induction of anesthesia and tracheal intubation with sevoflurane in adults. Anesthesiology. 1996;85:536–43. https://doi.org/10.1097/00000542-199609000-00012.
Masui K, Hagihira S. Equilibration rate constant, keo, to determine effect-site concentration for the Masui remimazolam population pharmacokinetic model in general anesthesia patients. J Anesth. 2022;36:757–62. https://doi.org/10.1007/s00540-022-03099-8.
Kodaka M, Okamoto Y, Handa F, Kawasaki J, Miyano H. Relation between fentanyl dose and predicted EC50 of propofol for laryngeal mask insertion. Br J Anaesth. 2004;92:238–41. https://doi.org/10.1093/bja/aeh033.
Obara S. Simulation of residual sedation effect of remimazolam: pharmacokinetic-pharmacodynamic simulation can be an additional standard anesthesia monitoring method. J Anesth. 2022;36:167–70. https://doi.org/10.1007/s00540-021-02963-3.
Doi M. Remimazolam. J Jpn Soc Clin Anesth. 2014;34:860–6. (in Japanese with English abstract).
Liu T, Lai T, Chen J, Lu Y, He F, Chen Y, Xie Y. Effect of remimazolam induction on hemodynamics in patients undergoing valve replacement surgery: a randomized, double-blind, controlled trial. Pharmacol Res Perspect. 2021;9:e00851. https://doi.org/10.1002/prp2.851.
Doi M, Morita K, Takeda J, Sakamoto A, Yamakage M, Suzuki T. Efficacy and safety of remimazolam versus propofol for general anesthesia: a multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J Anesth. 2020;34:543–53. https://doi.org/10.1007/s00540-020-02788-6.
Hasegawa G, Hirata N, Yoshikawa Y, Yamakage M. Differential effects of remimazolam and propofol on heart rate variability during anesthesia induction. J Anesth. 2022;36:239–45. https://doi.org/10.1007/s00540-022-03037-8.
Shin HY, Lim JA, Kim SH, Baek SW, Kim DK. Desflurane requirements for laryngeal mask airway insertion during inhalation induction. J Anesth. 2009;23:209–14. https://doi.org/10.1007/s00540-008-0730-3.
Burlacu CL, Gaskin P, Fernandes A, Carey M, Briggs L. A comparison of the insertion characteristics of the laryngeal tube and the laryngeal mask airway: a study of the ED50 propofol requirements. Anaesthesia. 2006;61:229–33. https://doi.org/10.1111/j.1365-2044.2005.04442.x.
Ashay NA, Wasim S, Anil TB. Propofol requirement for insertion of I-gel versus laryngeal mask airway: a comparative dose finding study using Dixon’s up-and-down method. J Anaesthesiol Clin Pharmacol. 2015;31:324–8.
Shafer SL, Varvel JR. Pharmacokinetics, pharmacodynamics, and rational opioid selection. Anesthesiology. 1991;74:53–63. https://doi.org/10.1097/00000542-199101000-00010.
Yumura J, Koukita Y, Fukuda K, Kaneko Y, Ichinohe T. Low dose of fentanyl reduces predicted effect-site concentration of propofol for flexible laryngeal mask airway insertion. J Anesth. 2009;23:203–8. https://doi.org/10.1007/s00540-008-0728-x.
Masui K, Stöhr T, Pesic M, Tonai T. A population pharmacokinetic model of remimazolam for general anesthesia and consideration of remimazolam dose in clinical practice. J Anesth. 2022;36:493–505. https://doi.org/10.1007/s00540-022-03079-y.
Wang H, Gao X, Wei W, Miao H, Meng H, Tian M. The optimum sevoflurane concentration for supraglottic airway device Blockbuster™ insertion with spontaneous breathing in obese patients: a prospective observational study. BMC Anesthesiol. 2017;17:156. https://doi.org/10.1186/s12871-017-0449-5.
Meng W, Kang F, Dong M, Wang S, Han M, Huang X, Wang S, Li J, Yang C. Remifentanil requirement for i-gel insertion is reduced in male patients with Parkinson’s disease undergoing deep brain stimulator implantation: an up-and-down sequential allocation trial. BMC Anesthesiol. 2022;22:197. https://doi.org/10.1186/s12871-022-01735-0.
Kim MK, Lee JW, Jang DJ, Shin OY, Nam SB. Effect-site concentration of remifentanil for laryngeal mask airway insertion during target-controlled infusion of propofol. Anaesthesia. 2009;64:136–40. https://doi.org/10.1111/j.1365-2044.2008.05707.x.
Hui JK, Critchley LA, Karmakar MK, Lam PK. Co-administration of alfentanil-propofol improves laryngeal mask airway insertion compared to fentanyl-propofol. Can J Anaesth. 2002;49:5508–12. https://doi.org/10.1007/BF03017932.
Li N, Chen Y, Ouyang B, Li G, Lin G, Li Y, Li T. The optimal bolus dose of sufentanil for satisfactory laryngeal mask airway (LMA) insertion conditions in Chinese pediatric patients: a prospective double-blind randomized controlled trial (CONSORT). Med (Baltim). 2019;98(10):e14711. https://doi.org/10.1097/MD.0000000000014711.
Acknowledgements
We thank co-research doctors of the Department of Anesthesiology and Intensive Care, Hamamatsu University School of Medicine. We thank Victoria Muir, PhD, from Edanz Group (https://www.jp.edanz.com/ac) for editing a draft of this manuscript.
Funding
Financial support was provided solely from departmental sources.
Author information
Authors and Affiliations
Contributions
YN helped conceive this study. YN and TK helped design this study. Patient recruitment and data collection were performed by HN. HN, MS and KM analyzed the data. HN wrote the first draft of the manuscript. TK edited the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Clinical Research Review Board of Hamamatsu University School of Medicine (Date 15 April 2021/No. CRB4180008).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nishimoto, H., Kurita, T., Shimizu, M. et al. Predicted effect-site concentrations of remimazolam for i-gel insertion: a prospective randomized controlled study. J Clin Monit Comput (2024). https://doi.org/10.1007/s10877-024-01135-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10877-024-01135-4