Abstract
To compare pulse oximetry performance during simulated conditions of motion and low perfusion in three commercially available devices: GE HealthCare CARESCAPE ONE TruSignal SpO2 Parameter, Masimo RADICAL-7 and Medtronic Nellcor PM1000N. After IRB approval, 28 healthy adult volunteers were randomly assigned to the motion group (N = 14) or low perfusion (N = 14) group. Pulse oximeters were placed on the test and control hands using random assignment of digits 2–5. Each subject served as their own control through the series of repeated pair-wise measurements. Reference co-oximetry oxyhemoglobin (SaO2) measurements from the radial artery were also obtained in the motion group. SpO2 readings were compared between the test and control hands in both groups and to SaO2 measurements in the motion group. Accuracy was assessed through testing of accuracy root-mean squared (ARMS) and mean bias. In the simulated motion test group the overall Accuracy Root Mean Square (ARMS) versus SaO2 was 1.88 (GE), 1.79 (Masimo) and 2.40 (Nellcor), with overall mean bias of − 0.21 (Masimo), 0.45 (GE), and 0.78 (Nellcor). In the motion hand, ARMS versus SaO2 was 2.45 (GE), 3.19 (Masimo) and 4.15 (Nellcor), with overall mean bias of − 0.75 (Masimo), − 0.01 (GE), and 0.04 (Nellcor). In the low perfusion test group, ARMS versus the control hand SpO2 for low PI was 3.24 (GE), 3.48 (Nellcor) and 4.76 (Masimo), with overall bias measurements of − 0.53 (Nellcor), 0.96 (GE) and 1.76 (Masimo). Experimental results for all tested devices met pulse oximetry regulatory and testing standards requirements. Overall, SpO2 device performance across the three devices in this study was similar under both motion and low perfusion conditions. SpO2 measurement accuracy degraded for all three devices during motion as compared to non-motion. Accuracy also degraded during normal to low, very low, or ultra low perfusion and was more pronounced compared to the changes observed during simulated motion. While some statistically significant differences in individual measurements were found, the clinical relevance of these differences requires further study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In 1974 in Japan, Takuo Aoyagi and Akio Yamanishi independently filed patents which became the foundational science for current pulse oximetry technologies [1]. Since then, pulse oximetry has become a widely recognized standard of care for numerous clinical applications where the monitoring of oxygen saturation, or oxyhemoglobin, is required to optimize outcomes. Pulse oximetry is likely the most commonly used medical device for both inpatient and outpatient care [2] and is associated with more rapid detection and treatment of respiratory compromise, particularly in the perioperative setting [3, 4].
Pulse oximetry (SpO2) technology requires an arterial pulse signal, light emitting diodes, and a photo detector. In clinical settings, an oximeter is used to transmit light with red and infrared wavelengths most commonly through the tissues of the finger or ear. The tissue absorbs much of the emitted light, while the remainder passes through the tissue to be measured by a light-sensitive photodiode. As oxygen saturation increases, the more infrared light is absorbed by oxyhemoglobin and the more red light is transmitted through. Conversely, as oxygen saturation decreases, the more red light is absorbed by deoxyhemoglobin and the more infrared light is transmitted through. The measured ratios of red to infrared light transmission by the photodiode allow for the calculation of the percentage fraction of oxygenated hemoglobin, resulting in a displayed clinical SpO2 reading [5,6,7]. While SpO2 values are noninvasive estimates of oxyhemoglobin levels, SaO2 levels provide direct measurements of oxyhemoglobin levels as collected through arterial blood samples. SaO2 measurements are considered the gold standard for oxyhemoglobin assessment [8].
Pulse oximeter accuracy may be impacted by multiple factors including, but not limited to, low perfusion, motion, skin pigmentation, dyshemoglobinemias, anemia, dyes, nail polish, and ambient light [8]. Low perfusion states, such as sepsis or cardiogenic shock, may decrease SpO2 accuracy because pulse oximetry requires a sufficient arterial pulse signal which is often decreased during these conditions. Patient movement, such as that associated with shivering or delirium, creates artifacts which interfere with SpO2 measurement and impact measurement accuracy. These inaccuracies place patients at risk for delayed or missed recognition of hypoxemia. Precise SpO2 readings are especially important for the safe care of critically ill patients.
Pulse oximetry inaccuracy related to skin pigmentation is currently being reevaluated by the United States Food and Drug Administration (FDA), who held a Medical Devices Advisory Committee meeting on pulse oximetry in November of 2022 [8]. This inaccuracy may have notable implications for the treatment of patients with varying skin pigmentation [6, 9, 10].
Pulse oximetry technology has improved over time to reduce measurement errors, including those caused by motion and low perfusion. The use of these newer algorithms has been shown to improve clinical performance by reducing both data dropout and false alarms [11, 12]. However, even with these improvements, studies performed in laboratory settings using either a high-fidelity simulator or healthy volunteers and simulated conditions demonstrate that motion and low perfusion continue to present challenges for measurement accuracy [13, 14]. While differences in the measurement accuracy of various pulse oximeters have been reported, no specific type or brand of pulse oximeter has been found to be superior overall [14, 15]. The combination of low perfusion and increased levels of skin pigmentation may pose additional challenges to the accuracy of pulse oximetry measurements [16].
Variations in pulse oximetry accuracy may be caused by hardware, software and algorithms, wireless connectivity, and other design elements which have been introduced to maximize signal quality and reliability across a variety of challenging clinical conditions. Thus, the purpose of this study was to evaluate and compare the accuracy of three currently available pulse oximeters: (GE HealthCare CARESCAPE ONE TruSignal SpO2 Parameter, Masimo RADICAL-7, Medtronic Nellcor PM1000N SpO2) under simulated conditions of motion and low perfusion in a group of healthy volunteers.
2 Methods
2.1 Design
This was a prospective, open-labeled comparative evaluation of three commercially available pulse oximeters: (GE HealthCare CARESCAPE ONE TruSignal SpO2 Parameter, Masimo RADICAL-7, Medtronic Nellcor PM1000N SpO2) under conditions of motion and low perfusion across four phases of oxygenation. This study was approved by the University of California, San Francisco Committee on Human Research (San Francisco, California) and written informed consent was obtained from all participants. The study design was aligned with ISO 80601-2-61:2017 and FDA Guidance for Pulse Oximeter Pre-Market Notification Submissions [17].
2.2 Study participants
Twenty-eight healthy adult (≥ 18 to < 50 years) volunteer subjects were enrolled. Inclusion criteria were good general health, non-smokers, and normal hemoglobin (≥ 10 g/dL). Exclusion criteria were obesity, serious systemic illness, diabetes, cardiovascular disease, pulmonary disease, Raynaud’s disease, clotting disorders, and pregnant or lactating females. Subject enrollment was designed to meet FDA guidance requirements of a minimum of two darkly pigmented subjects or 15% of the total pool, whichever is larger [17]. Skin pigmentation was categorized by the 6-level Fitzpatrick Scale [18, 19].
Half of the subjects (N = 14) were randomly assigned to the motion protocol and the other half (N = 14) were randomly assigned to the low perfusion protocol. A minimum threshold of measurement pairs was included in accordance with ISO 80601-2-61:2017. The 14-subject sample size for each protocol meets FDA requirements [FDA] for the study and is consistent with other published analyses of similar technologies [2, 13]. The study was not powered to undertake subgroup analysis.
2.3 Protocol
Subjects in both motion and low perfusion groups had three pulse oximeters placed on both a test hand (motion or low perfusion) and control hand (non-motion or normal perfusion). Pulse oximeters were randomly assigned to digits 2 to 5 on both test and control hands to mitigate for order bias.
Subjects were administered air–nitrogen–carbon dioxide mixtures with a voluntarily increased minute ventilation, with carbon dioxide added as needed to maintain normocapnia. The test administrator adjusted the inspired air–nitrogen–carbon dioxide mixture breath-by-breath to achieve a series of stable SaO2 plateaus at desired saturation levels. The stable saturation plateau was maintained for at least 60 s with SpO2 fluctuating by less than 2–3%. This method has been used in previous studies [13] and typically requires a period of time for the oxygen saturation to stabilize. The controlled desaturation study procedure followed the guidelines of pulse oximetry standard ISO 80601-2-61:2017: Annex EE.2 PROCEDURE for invasive laboratory testing on healthy adult volunteers (motion group) and Annex EE.3 PROCEDURE for non-invasive laboratory testing on healthy adult volunteers (low perfusion group). ISO 80601-2-61:2017:Annex EE.2 proposes to have ≥ 30 s plateau before blood sample.
2.4 Data collection
2.4.1 Motion testing
In the motion group, each subject had two control blood samples taken at the beginning of each experiment, while breathing room air. For each subject, desaturation was repeated six times to reach a low SpO2 plateau (SpO2 target 85–90%) with a period of high SpO2 plateau (approximately 92–100%) between each round. At each SpO2 plateau, a blood sample was taken and used to perform pair-wise comparisons of the test hand and control hand SpO2 measurements against the CO-oximeter SaO2.
Motion was induced palm down using a clenching technique, pressing and rubbing motion (CPR), palm up with twitching/clenching (T/C), and a tapping motion (Tap). Motion conditions were generated by the test subjects with variable intensity and frequency. Oximeters were recorded continuously to collect SpO2 readings across each saturation plateau. SpO2 readings were compared between the test and control hands and to simultaneous SaO2 measurements to assess accuracy. The motion methodology was adapted from a study by Tobin et al. [20] characterizing the motion artifact types in hospitalized patients. Subject generated motion was also used more recently in another study by Louie [13]. Compared to machine generated motion, this study method has more variability and is more clinically relevant as simulation of patient movement. To ensure that the motion conditions are approximately equal across the tested devices, the test subjects were observed during the testing and instructed to keep motion between sensors equal. To randomize the possible intensity differences between fingers, the sensors were rotated between fingers after three of six desaturation cycles for each subject.
Arterial blood was sampled (in total N = 248 GE, N = 250 Nellcor and Masimo) at each saturation plateau to obtain SaO2 values. Data are grouped into SaO2 ranges of 70–100, 80–90, and 90–100 to summarize pulse oximeter performance in various saturation groups. FDA guidelines for accuracy testing were used to measure at least 200 data points as paired SpO2−SaO2 observations balanced across each decadal range of SaO2 [17]. As previously mentioned, FDA guidance also recommends a sample size of at least 10 healthy subjects that vary in age and gender, with a range of skin pigmentation, including at least two darkly pigmented subjects or 15% of your subject pool, whichever is larger.
2.4.2 Low perfusion testing
In the low perfusion group, the multiple step desaturation method was used to collect the data pairs in SpO2 plateaus distributed evenly over the SpO2 accuracy range of 70–100%. For each subject, the stepwise desaturation process to achieve the 70% SpO2 level was repeated twice with a high SpO2 period and sensor rotation between. The target was to achieve ten SpO2 plateaus with each subject and in each SpO2 plateau to collect two test hand—control hand SpO2 data pairs.
Each subject’s left arm was submerged in an ice bath while the right arm was kept warm to serve as a control. In this group of healthy volunteers there were no expected baseline perfusion differences between the right and left arm, so the left arm was used in all subjects for consistency of experimental setup. Due to the time required to develop low perfusion in the experimental arm and the time that would have been required to recover that extremity to normal perfusion and immerse the opposite arm, rotation of the test and control arms was not feasible. The length of submersion was determined by the Perfusion Index (PI) as measured by the GE SpO2 device. PI is calculated as the ratio of pulsatile blood flow divided by the non-pulsatile blood flow times 100. Left arm cooling was performed until a PI value of less than 0.3% was reached, or a maximum of 60 min.
Pulse oximetry measurements were recorded continuously at each saturation plateau and SpO2 readings were compared between the test and control hands. PI values were recorded and grouped into five perfusion ranges: All, normal (PI ≥ 1.0), low (0.3 ≤ PI < 1.0), very low (0.1 ≤ PI < 0.3), and ultra low (PI < 0.1) to allow us to assess pulse oximeter performance across the various perfusion groups. The number of datapoints was equal across the subjects.
2.5 Data analysis
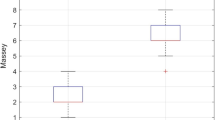
Statistical analysis was conducted with SAS 9.4. Descriptive data for comparison included the Accuracy Root Mean Square (ARMS) and bias. In the motion group, ARMS and bias was calculated as SpO2 minus the SaO2 reference value, with SaO2 serving as the reference. In the low perfusion group, the control SpO2 served as the reference. ARMS was calculated as the square root of the mean of the squared difference between test and reference values (Fig. 1).
ANOVA with post-hoc Dunnett test was used for comparison of mean biases. The homogeneity of ARMS was tested with Levene’s test. Bland–Altman method was used to visualize the relationship between tested SpO2 measurements against the reference method and to determine limits of agreement.
2.6 Materials
All study devices (GE HealthCare CARESCAPE ONE TruSignal SpO2 Parameter, Masimo RADICAL-7, Medtronic Nellcor PM1000N SpO2) were CE marked and 510(k) cleared by the US FDA. Disposable adhesive sensors were used to prevent sensor displacement.
In the motion group, a 22-gauge radial arterial catheter was used for sampling reference co-oximetry oxyhemoglobin (SaO2) measurements on the control extremity. Blood gas analysis to determine SaO2 was performed with the ABL-90 multi-wavelength oximeter (Hemoximeter, Radiometer, Copenhagen, Serial 1393-090R0359N0002). In the low perfusion group, PI values were collected using the GE SpO2 device.
Ethically, to minimize the study risks for the subjects an arterial line was used only in the motion group. Arterial blood samples were collected to allow for comparison of device accuracy against the gold standard SaO2 in both non-motion and motion conditions. In the low perfusion group, the same secondary standard pulse oximeter device and model was used for SpO2 measurements on both the warm control hand and the cooled test hand, as an alternative to invasive testing, pulse oximetry standard ISO 80601-2-61:2017 annex EE.3 proposes a non-invasive comparison of SpO2 device accuracy through a validated secondary standard pulse oximeter. Calibration of the secondary oximeter is directly traceable to a CO-oximeter and thus serves as the transfer standard.
3 Results
3.1 Demographics
In the motion group (N = 14), 5 women and 9 men were included, with an age range of 24–43 years and a mean age of 28.1 (SD = 5.2) years. Skin tones varied by the Fitzpatrick scale as Type II (N = 1), Type III (N = 6), Type IV (N = 5), Type V (N = 1), and Type VI (N = 1). Ethnicity varied Asian (N = 5), Caucasian (N = 5), Hispanic (N = 2), Black (N = 1), and Multiethnic (N = 1).
In the low perfusion group (N = 14), 9 women and 5 men were included, with an age range of 20–48 and a mean age of 28.7 (SD = 7.8) years. Skin tones varied by the Fitzpatrick scale as Type II (N = 4), Type III (N = 5), Type IV (N = 3), Type V (N = 1), and Type VI (N = 1). Ethnicity varied Asian (N = 4), Caucasian (N = 6), African American (N = 1), and Multiethnic (N = 3).
3.2 Non-motion results-control SpO2 vs. SaO2
The bias and ARMS results of the non-motion, control SpO2 sensors versus reference SaO2 are presented in Table 1. and Fig. 2. For the SaO2 range of 70–100, mean bias was less than 1 for each of the three devices, ARMS was lowest for Masimo at 1.79 and highest for Nellcor at 2.40. For SaO2 range of 80–90, mean bias was lowest for Masimo at 0.28 and highest for Nellcor at 1.68. ARMS was lowest for Masimo at 2.00 and highest for Nellcor at 2.89. For SaO2 range of 90–100, mean bias was less than 1 for each device, ARMS ranged from 1 to 2 for all devices. No significant differences in ARMS between devices were found in any of these comparisons. Mean bias measures were significantly different for each analysis range (P < 0.0001).
3.3 Motion results-test conditions SpO2 vs. SaO2
The bias and ARMS results of the test SpO2 sensors during motion versus reference SaO2 are presented in Table 2 and Fig. 3. For the whole covered SaO2 range of 70–100, mean bias was less than 1 for each device, with ARMS lowest for GE at 2.45 and highest for Nellcor at 4.15. For SaO2 range of 80–90, mean bias was less than 1 for GE and Masimo and 1.07 for Nellcor. ARMS was lowest for GE at 3.01 and highest for Nellcor at 5.31. For SaO2 range of 90–100, mean bias was less than 1 for each device, ARMS was lowest for GE at 2.06 and highest for Nellcor at 3.29. Significant differences in bias were observed in all analyzed ranges (P < 0.05). Significant differences in ARMS were observed in the 70–100 range across all devices (P < 0.005) and between GE and Nellcor at each analyzed range (P < 0.05).
3.4 Low perfusion results-test SpO2 vs. control SpO2
The bias and ARMS results of the test SpO2 sensors versus reference SpO2 sensor are presented in Table 3. and Fig. 4. For all perfusion ranges, mean bias was the lowest for Nellcor at − 0.35 and greatest for Masimo at 1.62 (p < 0.0001). ARMS was lowest for GE at 3.26 and highest for Masimo at 4.30 (p = NS). For low PI, the mean bias was the lowest for Nellcor at − 0.53 and highest for Masimo at 1.76 (p < 0.0001). ARMS was the lowest for GE at 3.24 and highest for Masimo at 4.76 (p = NS). At very low PI, mean bias was the lowest for Nellcor at − 0.29 and highest for Masimo at 2.36, ARMS was lowest for GE at 4.52 and highest for Nellcor at 5.55 (p = NS for both mean and ARMS).
4 Discussion
This study adds to the existing body of evidence on pulse oximeter performance under conditions of motion and low perfusion. We believe this is the first study to induce significant levels of low perfusion using an ice bath test method.
The non-motion test results showing the measurements of the control hand versus SaO2 shows minimal bias in all three devices and the ARMS showed no significant differences. In this scenario, the clinical challenges to SpO2 measurement accuracy are minimal, simulating pulse oximetry measurement in a patient with normal perfusion and little to no motion, such as during elective procedural care or outpatient medicine. This finding is consistent with previous research, which has found similar performance across most SpO2 devices [14, 15].
An ongoing concern is the potential impact of skin pigmentation levels on SpO2 measurements, which have been previously reported [9, 10, 21, 22]. While we included subjects with a range of skin pigmentation, this study was not designed to specifically assess the impact of skin pigmentation on SpO2 performance, and thus this remains an area for future study.
The finding of two oximeters (Nellcor and GE) having reduced bias during motion versus non-motion conditions for the SaO2 range of 70–100 was unexpected, since performance is normally decreased during motion. Because the differences were small, the observation may be explained by limitations in measurement accuracy. The mean bias of − 0.75 observed with Masimo during motion conditions, versus a mean bias of 0.78 with Nellcor during non-motion conditions, suggests that this degree of error may fall within the accuracy limits of SpO2 performance. More importantly, a bias less than 1 may not be clinically significant when interpreted with additional data describing the clinical condition of the patient.
A study by Louie et al., found the ARMS error greater than 3% in motion test conditions in all devices except for Nihon Kohden [13]. In the 70–100% saturation range used in this study, the GE device had an ARMS error of 2.45, while Nellcor and Masimo had ARMS of 4.15 and 3.19 respectively (P < 0.005). However, overall the SpO2 performance was similar among the three devices and is consistent with the findings reported by Louie.
The low perfusion test conditions resulted in greater performance degradation and larger mean bias and ARMS values. The normal PI of ≥ 1.0 is associated with mean bias levels less than one, but ARMS values ranged from 1.78 (Nellcor) to 2.14 (GE). In this study normal PI is defined as ≥ 1.0, while in a previous study PI values < 2 were considered representative of poor perfusion [13].
In the low and ultra low PI ranges, significant differences in mean bias were observed across devices. In the very low PI range, no significant differences were evident. At ultra low levels of perfusion, Masimo (N = 9) and Nellcor (N = 10) experienced a number of missing values while GE had no missing values. The increase in proportion of missing values with ultra low PI, suggests that a threshold for pulse oximeter performance may have been reached although additional study is required to evaluate further. However, due to the low number of low and ultra low PI samples, no conclusions can be reached regarding relative pulse oximeter performance. Additional studies using larger patient populations and data sets which include more low and ultra low PI samples are required compare performance across different SpO2 devices.
Loss of pulse oximetry signals due to low perfusion is a clinical challenge requiring additional actions to estimate arterial oxygen levels and hemoglobin saturation. If the signal is lost from a finger, probes may be applied on alternative anatomic locations such as toes, ears, buccal mucosa, or nares. Invasive measurement via arterial blood gas is a clinical option when the pulse oximetry signal is not reliable. However, invasive measurement is associated with increased risk of patient morbidity due to line placement, line dislodgement, repeated blood sampling, delays due to the requirement to transport and run samples, anemia if repeated measurement is required, and costs from supplies and equipment use [23,24,25].
The motion testing group assessed pulse oximetry performance over a wide range of clinically relevant conditions. Findings demonstrated similar performance of all three SpO2 devices even though some statistically significant differences in bias and ARMS were observed. The accuracy of SpO2 measurement during low perfusion conditions showed a greater degree of degradation when compared to normal perfusion, with statistically significant differences in bias found primarily in the low perfusion measurements. These study findings highlight the limitations of pulse oximetry technologies which are dependent on pulsatile blood for accurate measurement. Since conditions of low perfusion are common in the clinical setting, it is important to recognize that pulse oximeter measurements without the context of other relevant clinical data, are often not sufficient to guide diagnostic and therapeutic clinical decisions.
4.1 Limitations
As this study was conducted in a controlled laboratory setting, it is unlikely that we were able to fully replicate or adequately represent device performance in the actual clinical environment. This study was conducted using healthy volunteer subjects without significant illness or comorbidity, which is not representative of a typical patient population, particularly in acute care. Moreover, our sample for subjects with darker skin pigmentation was small, limiting the use of these results in this patient population. The study was not powered to examine the impact of motion or low perfusion on any patient subgroups. Finally, because of the low number of observations of ultra low PI (N = 21), no meaningful conclusions of SpO2 comparative performance can be made at this PI strata.
5 Conclusion
The overall finding from this study is that performance of all three SpO2 devices was similar across simulated motion and low perfusion conditions. Consistent with previous research on the impact of motion, the SpO2 measurement accuracy degraded for all three devices when compared to non-motion controls. For all three devices, accuracy also degraded as the perfusion index was reduced.
Pulse oximetry innovations to improve the quality, accuracy, and consistency of SpO2 measurements during clinical use are needed to improve patient safety. Continued technology development and additional studies are required to further improve SpO2 measurement accuracy and mitigate for limitations of use during motion, low perfusion, and in patients with darker skin pigmentation.
References
Miyasaka K, Shelley K, Takahashi S, Kubota H, Ito K, Yoshiya I, et al. Tribute to Dr. Takuo Aoyagi, inventor of pulse oximetry. J Anesth. 2021;35(5):671–709. https://doi.org/10.1007/s00540-021-02967-z.
Bickler P, Tremper KK. The pulse oximeter is amazing, but not perfect. Anesthesiology. 2022;136(5):670–1. https://doi.org/10.1097/ALN.0000000000004171.
Severinghaus JW. Takuo Aoyagi: discovery of pulse oximetry. Anesth Analg. 2007;105(6):S1–S4. https://doi.org/10.1213/01.ane.0000269514.31660.09.
Taenzer AH, Pyke JB, McGrath SP, Blike GT. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. J Am Soc Anesthesiol. 2010;112(2):282–7. https://doi.org/10.1097/ALN.0b013e3181ca7a9b.
Wick KD, Matthay MA, Ware LB. Pulse oximetry for the diagnosis and management of acute respiratory distress syndrome. Lancet Respir Med. 2022;10(11):1086–98. https://doi.org/10.1016/S2213-2600(22)00058-3.
Clark AP, Giuliano K, Chen HM. Pulse oximetry revisited: “but his O(2) sat was normal! Clin Nurse Spec. 2006;20(6):268–72. https://doi.org/10.1097/00002800-200611000-00004.
Jubran A. Pulse oximetry. Crit Care. 2015;19(1):272. https://doi.org/10.1186/s13054-015-0984-8.
Food and Drug Administration. FDA executive summary: review of pulse oximeters and factors that can impact their accuracy. United States Food and Drug Administration 2022)https://www.fda.gov/media/162709/download
Sjoding MW, Iwashyna TJ, Valley TS. Change the framework for pulse oximeter regulation to ensure clinicians can give patients the oxygen they need. Am J Respir Crit Care Med. 2022. https://doi.org/10.1164/rccm.202209-1773ED.
Fawzy A, Wu TD, Wang K, Robinson ML, Farha J, Bradke A, et al. Racial and ethnic discrepancy in pulse oximetry and delayed identification of treatment eligibility among patients with COVID-19. JAMA Intern Med. 2022;182(7):730–8. https://doi.org/10.1001/jamainternmed.2022.1906.
Giuliano KK, Higgins TL. New-generation pulse oximetry in the care of critically ill patients. Am J Crit Care. 2005;14(1):26–37. https://doi.org/10.4037/ajcc2005.14.1.26.
Petterson MT, Begnoche VL, Graybeal JM. The effect of motion on pulse oximetry and its clinical significance. Anesth Analg. 2007;105(6):S78–S84. https://doi.org/10.1213/01.ane.0000278134.47777.a5.
Louie A, Feiner JR, Bickler PE, Rhodes L, Bernstein M, Lucero J. Four types of pulse oximeters accurately detect hypoxia during low perfusion and motion. Anesthesiology. 2018;28(3):520–30. https://doi.org/10.1097/aln.0000000000002002.
Ganesh Kumar M, Kaur S, Kumar R. Laboratory evaluation of performance of pulse oximeters from six different manufacturers during motion artifacts produced by Fluke 2XL SpO(2) simulator. J Clin Monit Comput. 2022;36(4):1181–91. https://doi.org/10.1007/s10877-021-00747-4.
Poorzargar K, Pham C, Ariaratnam J, Lee K, Parotto M, Englesakis M, Chung F, Nagappa M. Accuracy of pulse oximeters in measuring oxygen saturation in patients with poor peripheral perfusion: a systematic review. J Clin Monit Comput. 2022;36(4):961–73. https://doi.org/10.1007/s10877-021-00797-8.
Gudelunas MK, Lipnick M, Hendrickson C, Vanderburg S, Okunlola B, Auchus I, et al. Low perfusion and missed diagnosis of hypoxemia by pulse oximetry in darkly pigmented skin: a prospective study. medRxiv. 2022. https://doi.org/10.1101/2022.10.19.22281282.
Food and Drug Administration Center for Devices and Radiological Health. Pulse oximeters—premarket notification submissions [510(k)s]. Washington, DC: U.S. Department of Health and Human Services; 2013. https://www.fda.gov/media/72470/download.
Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124(6):869–71. https://doi.org/10.1001/archderm.124.6.869.
Gupta V, Sharma VK. Skin typing: Fitzpatrick grading and others. Clin Dermatol. 2019;37(5):430–6. https://doi.org/10.1016/j.clindermatol.2019.07.010.
Tobin RM, Pologe JA, Batchelder PB. A characterization of motion affecting pulse oximetry in 350 patients. Anesth Analg. 2002;94(1):S54–S61.
Burnett GW, Stannard B, Wax DB, Lin HM, Pyram-Vincent C, DeMaria S, et al. Self-reported race/ethnicity and intraoperative occult hypoxemia: a retrospective cohort study. Anesthesiology. 2022;136(5):688–96. https://doi.org/10.1097/ALN.0000000000004153.
Okunlola OE, Lipnick MS, Batchelder PB, Bernstein M, Feiner JR, Bickler PE. Pulse oximeter performance, racial inequity, and the work ahead. Respir Care. 2022;67(2):252–7. https://doi.org/10.4187/respcare.09795.
Brzezinski M, Luisetti T, London MJ. Radial artery cannulation: a comprehensive review of recent anatomic and physiologic investigations. Anesth Analg. 2009;109(6):1763–81. https://doi.org/10.1213/ANE.0b013e3181bbd416.
Hess D. The AARC (American Association for Respiratory Care) clinical practice guidelines. Respir Care. 1991;36(12):1398–401.
Melanson SE, Szymanski T, Rogers SO, Jarolim P, Frendl G, Rawn JD, et al. Utilization of arterial blood gas measurements in a large tertiary care hospital. Am J Clin Pathol. 2007;127(4):604–9. https://doi.org/10.1309/elh5bpq0t17rrk0m.
Acknowledgements
None.
Funding
Funding for this work was provided by GE HealthCare.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception, design, material preparation, data collection and analysis. The first draft of the manuscript was written collaboratively by all authors, and all authors provided edits and comments on all subsequent drafts. All authors read and approved the final version of the revised manuscript.
Corresponding author
Ethics declarations
Competing interests
JWB and SL are full time employees of GE Healthcare. KKG and RB are paid consultants for GE Healthcare.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the University of California, San Francisco Committee on Human Research (San Francisco, California).
Consent to participate
Written informed consent was obtained from all participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Giuliano, K.K., Bilkovski, R.N., Beard, J. et al. Comparative analysis of signal accuracy of three SpO2 monitors during motion and low perfusion conditions. J Clin Monit Comput 37, 1451–1461 (2023). https://doi.org/10.1007/s10877-023-01029-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-023-01029-x