Abstract
The poor design of conventional auditory medical alarms has contributed to alarm desensitization, and eventually, alarm fatigue in medical personnel. This study tested a novel multisensory alarm system which aims to help medical personnel better interpret and respond to alarm annunciation during periods of high cognitive load such as those found within intensive care units. We tested a multisensory alarm that combined auditory and vibrotactile cues to convey alarm type, alarm priority, and patient identity. Testing was done in three phases: control (conventional auditory), Half (limited multisensory alarm), and Full (complete multisensory alarm). Participants (N = 19, undergraduates) identified alarm type, priority, and patient identity (patient 1 or 2) using conventional and multisensory alarms, while simultaneously completing a cognitively demanding task. Performance was based on reaction time (RT) and identification accuracy of alarm type and priority. Participants also reported their perceived workload. RT was significantly faster for the Control phase (p < 0.05). Participant performance in identifying alarm type, priority, and patient did not differ significantly between the three phase conditions (p = 0.87, 0.37, and 0.14 respectively). The Half multisensory phase produced the lowest mental demand, temporal demand, and overall perceived workload score. These data suggest that implementation of a multisensory alarm with alarm and patient information may decrease perceived workload without significant changes in alarm identification performance. Additionally, a ceiling effect may exist for multisensory stimuli, with only part of an alarm benefitting from multisensory integration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
* Equal contribution.
1 Introduction
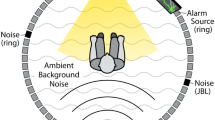
Intensive care units (ICUs) are high-consequence settings with constant activity requiring attention by medical personnel. In these settings, it is imperative that medical personnel receive efficient and accurate patient alarms for optimal care to be delivered. However, this necessity for patient alarm notification has resulted in a saturated auditory environment with unnecessarily loud and often false alarms [1,2,3]. In fact, research has shown that up to 85–99% of clinical alarms are false [4]. The alarm environment of frequent false and loud alarms has resulted in cognitive overload in medical personnel that manifests as alarm fatigue and results in missed alarms, even patient death [1,2,3,4,5,6].
Alarm fatigue, a form of sensory desensitization, has resulted from an overabundance of disruptive, false, and uninformative medical alarms [4,5,6,7]. This desensitization has led to medical personnel ultimately disregarding patient alarms due to the repetitive, non-actionable nature over a long-time course [3, 8]. It has been shown that for critical care nurses that the effects of divided attention and slow rate of IEC 60601-1-8, melodic alarm learning with persistent errors lead to the aforementioned issues as a result of alarm fatigue [9]. In another study, anesthesiologists under a high visual attentional load combined with consistent auditory noise had up to a 17% decline in task performance [5]. This is all to demonstrate that the current, conventional auditory alarm environment within the ICU is designed to alert personnel through generalized auditory annunciations, but this comes at the cost of positive predictive value. Consequently, alarm fatigue has been named a top safety priority within the past decade according to the 2022 Joint Commission, an organization that focuses on accrediting more than 20,000 health care organizations within the United States[6].
Alarms often exceed WHO volume recommendations (< 50 dB, often comparable to a human scream or infant’s cry) which negatively affect both medical personnel as well as patient [7]. In severe cases, these frequent alarms can contribute to the development of Post-Intensive Care Syndrome in patients, which is a collection of ailments that follow patients home after being discharged from and intensive care facility [10]. These symptoms can often range from a wide variety of physical problems to mental symptoms very similar to post-traumatic stress disorder. Fortunately, research has shown that quieter alarms, up to 11dB quieter than hospital background noise at 60 dB, result in the same simulated performance as alarms much louder [1]. A possible solution to this alarm problem is using alarms which integrate more than just the auditory sense to convey information.
Multisensory alarms integrate senses beyond that of hearing to reduce the unisensory burden [11]. The ICU setting is a high-consequence setting where cognitive processing and demand must be optimized, and therefore, alarms should be designed with cognitive load in mind [12]. Burdick et al. demonstrated a higher accuracy of alarm identification with the use of multisensory (auditory, vibrotactile, and visual) alarms, compared to conventional [11]. Fleishman et al. 2021 demonstrated that critical alert detection was faster with a multisensory (visual and auditory) alarm, compared to unisensory [13]. Both studies suggest that multisensory alarm systems had the potential to reduce the auditory burden associated with auditory alarms, as well as allow medical personnel to identify events more accurately and more rapidly when multiple, cognitively demanding sensory stimuli were present. This study aims to build further on these studies by investigating if there is a sensory processing ceiling effect to multisensory integration with vibrotactile stimuli.
Vibrotactile stimuli are used as an industry standard in areas such cell phones and the automotive industry. Vibrotactile devices in the automotive industry are useful in alerting drivers to present dangers when performing lane changes [14, 15]. Vibrotactile stimuli is commonly used cellular devices, automobiles, and even medicine, with laparoscopic surgical tools. When discussing the design of laparoscopic tools with surgeons, Cao et al. demonstrated that vibrotactile feedback enhanced performance, at the cost of speed, when completing simulated surgical skills [16]. Outside of medicine, devices such as smart watches have successfully integrated vibrotactile notifications without an audible cue. Furthermore, wearable vibrotactile devices have the potential to be specifically tailored to route physiological information from specific patients to specific personnel [17]; thereby, reducing the need for medical personnel to determine if an auditory alarm is relevant to their patient. This reduction of auditory alarm burden may help reduce noise pollution in the ICU and thereby reduce alarm desensitization.
With the current limitations of uninformative conventional alarms, the use of multisensory alarm systems using auditory and vibrotactile stimuli may lead to improved medical personnel reaction times, patient outcomes, and overall medical personnel efficiency and safety. In this study, we present a multisensory (auditory and vibrotactile) alarm design with varying vibrotactile alarm integration modalities and investigate participant reaction time and accuracy of alarm identification, compared to conventional unisensory alarms.
2 Methods
The research was approved by Vanderbilt University Medical Center Institutional Review Board (ID# 200,195). Participants (N = 19) were undergraduate students at Vanderbilt University and compensated with partial course credit or approved financial compensation of $20. Participants were recruited through classroom announcements and campus-wide advertising. Based on a power analysis, we determined that this experiment required at least 20 participants for adequate analysis. Recruitment resulted in greater than 20 participants; however, due to COVID-19 pandemic restrictions and study interruption, 19 participants were able to complete the experiment in the allotted time for data collection.
Materials
This study was based on an experimental set of code created, tested, and finalized on the latest versions of Microsoft Visual Studio (Redmond, WA). This experiment, and the accompanying visual studio code, was run on an Acer Spin 5 laptop (New Taipei City, Taiwan). We used C-2 tactors controlled by a central control unit designated as EAI_UC_98 from Engineering Acoustics Incorporated (EAI, Casselberry, Florida). The headphones utilized to convey auditory continuous tasks and auditory alarms were Audio-Technica over the ear headphones, designated ATH-M30x Professional Monitor Headphones (Tokyo, Japan).
Vibrotactile device placement
The multisensory alarm device utilized the EAI tactors to convey a series of vibrational sequences, or “cues,” which corresponded to specific alarms (described below). These cues were dispersed through the EAI tactors, and participants placed the vibrotactile alarm device on their lower left leg using the custom Velcro strap (example shown in supplemental Fig. 1).
3 Procedure
This study tested participants in 3 phases (control, multisensory half, and multisensory full) with 2 different cognitive tasks (visual and auditory, described below). In each phase, participants were played varying forms of an alarm and identified the: alarm type (vital sign or mechanical ventilator), patient (patient 1 or 2) and the alarm priority (high or medium). Participant total trials per phase varied from 296 to 393, due to the varying number of total patients for each modality combination. The overall contact time for each participant was approximately within the range of 90–120 min.
Phase design is described in Table 1 below. In phase 1 (control) participants were presented with conventional, unisensory auditory alarms to identify patient and alarm type, while completing a cognitive task. In phase 2 (multisensory half), the procedure was the same as phase 1, but participants were presented with conventional, unisensory auditory alarms to identify patient and alarm type, and vibrotactile stimuli to identify patient, while completing a cognitive task. In phase 3 (multisensory full), the procedure was the same as phase 1 and 2, but participants were presented with auditory alarms and vibrotactile stimuli to identify patient and alarm type, while completing a cognitive task.
Participants completed all phases while simultaneously completing either a visual or auditory cognitive task. After completion of the phases with one cognitive task, they completed the 3 phases with the other cognitive task. The order of phases and cognitive tasks were randomized for each participant prior to assessment.
Performance was measured based on reaction time (RT) and accuracy of identifying patient identity and alarm type. RT was the time from alarm initiation to participant response.
Participants completed phase 1–3 with both auditory and visual cognitive task.
Training
Prior to data collection, participants had 5 min to practice each cognitive task (auditory and visual), listen to each auditory alarm, and experience the vibrotactile cues. Participant performance on cognitive task was graded as correct (green light on screen), or incorrect (red light on screen). No visual or audible feedback ques were given for alarm selection task performance. This 5-minute block was long enough to expose participants to all aspects of the study, but not long enough to exhaust participants.
Auditory and Vibrotactile cues: We used standard auditory alarms, per International Electrotechnical Commission standard (IEC 60601-1-8) [18] as our auditory alarms. Auditory alarms were annunciated twice with 7 s in between. Additionally, each set of annunciations had a coded window of time in which they would play (16–24 s range). For auditory alarms, the annunciation consisted of designated alarm type and priority tones with volume distribution detailing patient identification (approximately 70/30 splits with louder in left ear denotes patient 1, louder in right denotes patient 2). The vibrotactile stimulus was designed to signal 2 pieces of information: patient identity (Fig. 1 shown in red/blue), and which alarm type was being triggered (Fig. 1 shown in black). Patient identity vibrotactile stimulations were used in phase 2 and 3, and alarm type vibrotactile stimulations were used in phase 3. The vibrotactile stimulus was combined with auditory alarms which also signaled alarm type and patient information.
Cognitive Task
This study utilized two altered versions of Wayne Kirchner’s N-back task [19], a validated method of stimulating cognitive workload and working memory [20]. This task was administered as either auditory or visually, and participants responded on the laptop. The N-back task is a well-accepted method of simulating cognitive workload and tasking working memory, which has been used in previous literature to simulate the clinical cognitive workload [19]. Performance on the cognitive task was not reported in our results, as this was a feasibility study for multisensory design.
4 National Aeronautics and Space Administration – Task load index (NASA-TLX)
At the end of each phase, participants completed a NASA-TLX survey, which is an accredited multidimensional assessment tool which rates perceived subject workload (Colligan et al. 2015) [21]. For each phase, participants provided qualitative feedback about their overall perceived mental, physical, temporal demands, as well as their perceived success and effort. The assessment consists of 11 questions for participants to score on a 1–7 scale (1 being very low on the scale and 7 being very high on the scale, though was reversed for “perceived success” in which 1 was very high and 7 was very low). The NASA-TLX also measures overall perceived workload, which is a summation of the five categories assessed. Category descriptions are provided in supplemental Table 1.
5 Performance
Participant performance was assessed by accurate identification of alarm type, priority, and patient. Performance was also assessed by RT. We conducted a secondary analysis on error rates. Overall identification errors were categorized as error in alarm type, patient localization and priority. Alarm type errors occurred when participants chose the correct patient, but the incorrect alarm type. Patient identification errors occurred when participants chose the incorrect patient, but the correct alarm. Alarm priority errors occurred when participants chose the correct alarm type but the incorrect priority meaning they chose “high” priority vs. “medium” priority (Reference Fig. 2. for high vs. medium buttons). We also analyzed missed responses which were instances when participants did not select any response before the next alarm was triggered, which was a time window coded to be any whole number value randomly selected within a 16–24 s range.
5.1 Statistical analysis
For primary participant performance analysis, generalized linear mixed models were used to compare performance by phase type. For reaction time, a linear mixed model was used to analysis the reaction time due to repeat measurement of participants. The dependent variable was ln(rt) in order to use linear model. The independent variables were – phase (Control, Multisensory Full or Multisensory Half), cognitive task condition (visual or auditory) and the interaction between them. Statistical significance was set at an alpha of 0.05. For perceived cognitive workload analysis, we used a qualitative assessment of NASA-TLX. Differences were assessed by ANOVA. For our secondary analysis, we analyzed errors in alarm type and patient localization and missed responses. Errors with combinations of categories were tallied and placed in their respective categories, i.e., missed alarm type, missed patient identification, and missed alarm priority. Differences were assessed by qualitative comparison.
6 Results
RT was significantly faster for the control phase (p < 0.05). Accuracy of alarm type identification did not differ significantly between the 3 phase conditions (p = 0.87; Table 2). Accuracy of patient identification did not differ significantly between the 3 phase conditions (p = 0.14; Table 2). Accuracy of alarm priority identification did not differ significantly between the 3 phase conditions (p = 0.37; Table 2). The average RT for the alarm was 3.66 s (sec).
NASA-TLX
Using a Raw NASA-TLX based questionnaire, the half vibrotactile alarm modality had the lowest perceived workload, and Control had the highest (Table 3). In addition, the Half modality was perceived to be the most successful due to the mean mental demand score (4.14; Table 3) being lower than both the control (5.00; Table 3) and the full (4.43; Table 3) alarm modality. Additionally, the half modality had the lowest overall perceived workload score (15.71; Table 3). The Full alarm modality was perceived to have the lowest physical demand, temporal demand, and perceived effort.
7 Secondary analysis: Error
There were 279 errors across the three alarm modalities. The highest percentage of errors were to alarm type errors (49.5%, Table 4). The alarm type error rate was lowest for the Control phase (47.8%, Table 4). Patient identification errors accounted for 12.2% of selection errors. The patient identification error rate was lowest for the Half phase (9.0%, Table 4). Alarm priority errors accounted for 16.5% of errors, with the most during Control phase. There were 13 (4.6%) missed responses, which occurred more often with the Half phase (Table 4). The additional 61 (21.8%) of errors were due to mixed errors (ex. errors in both alarm type and priority).
8 Discussion
This study investigated the potential benefit of a novel multisensory alarm device which incorporates both auditory and vibrotactile cues to convey vital patient information to medical personnel. The multisensory alarm system used in this investigation combined auditory and vibrotactile cues to potentially decrease cognitive workload and improve accuracy of alarm identification, while in a simulated cognitive environment. Overall, our feasibility study demonstrated a perceived decrease in overall workload without significant impact on participant performance.
In this experiment, The Half multisensory phase performed the best in alarm identification, suggesting that it was the most optimal balance of stimuli to both communicate patient information and ease translation. The Full multisensory phase did not perform as well as Half, suggesting a possible ceiling effect to multisensory stimuli. The Control phase yielded the quickest responses when compared with the remaining two modalities. Overall, accuracy did not falter with the new modalities, compared to Control, but perceived workload was less; indicated that new alarms may not hurt performance and may help with workload.
The possible ceiling effect described in our results for the Full modality may be explained by the neuroscience principle of inverse effectiveness. The inverse effectiveness principle describes that the magnitude of multisensory enhancement (expressed as a proportion of the best unisensory response), is inversely proportional to the efficiency of a unisensory specific stimuli [22]. In this case, the principle of inverse effectiveness explains how the multisensory stimuli used in our Full modality may have overstimulated the user by communicating all of the alarm information via multisensory stream, rather than simply alerting to a change [22]. Additionally, Stein et al.’s work on development of multisensory integration suggest that multisensory integration is of the most value when detecting stimuli that are inherently weakly effective on their own, and in the presence of both sensory and neural noise [23].
In our study, the Full modality paired vibrotactile cues with all aspects of the auditory alarm: alarm intensity, type, and patient identification. The Half modality only paired vibrotactile cues with patient identity. The Half modality utilized Stein et al.’s approach of adding a meaningful second stimuli along to strength response, without the generalized addition of vibrotactile cues with all auditory stimuli, as Fetsch et al. caution against [22]. By efficiently delivering patient identity information, the user can focus more cognitive load toward discerning alarm intensity and type. Therefore, these data demonstrate a strategic balance of multisensory stimuli to promote notification without contributing to sensory saturation.
In addition to the quantitative results of this experiment’s impact on the medical alarm environment, our results demonstrate a subjective lower perceived workload for the Half modality phase. As described in depth by Deb and Claudio, alarm fatigue is a major cause of staff deterioration, so the need to innovate and optimize the medical alarm for alarm delivery and user workload is a priority for clinical safety and efficiency [24]. Participant NASA-TLX scores demonstrate the Half phase to decrease workload, compared to the control phase, though not significantly. NASA-TLX is a verified assessment tool [21], but there is no gold standard for measuring cognitive workload in the current literature. Additionally, the power calculations for this experiment were done to optimize comparison of alarm identification, rather than perceived workload assessment. As such, the raw differences showing the Half phase with decreased workload should not be entirely discounted and are a meaningful contribution to our results and device function. With no significant differences in alarm and patient identification performance, the Half modality has comparable performance to control, with a benefit to user experience. These findings demonstrate the opportunity to use multisensory alarms to foster a better user workload.
Additionally, it is important to remember the purpose of alarms is to not miss certain clinical changes and events, making our assessment of error rates particularly intriguing for future alarm design. Our results demonstrated that alarm type identification had the highest rates of error. This is a known and consistent challenge in alarm research, where various medical alarms are hard to discriminate and identify [25]. Auditory icons were created with this in mind and will be an important integration to future designs [26]. Clearly, it is important for alarm devices to continue innovating how these variables are communicated, and have it done so in a clear manner. In addition to alarm engineering, clinical considerations, specifically from the nursing perspective are critical to consider for design after this feasibility study. Some future considerations include nursing threshold personalization, design for shift-long comfort, integration of a nursing decision making function. While current alarms are designed to similar alert to an event, the future of alarms should be to integrate and elevate the level of healthcare and patient safety that a clinician can provide.
Overall, our multisensory alarm demonstrated some benefit to workload, and equivalent alarm identification accuracy. We believe our results show a possible ceiling effect to multisensory alarms where the benefit of multisensory notification can decline with too much information communicated through a multimodal alarm. Fortunately, our study did identify a benefit to user workload, which is an important aspect to seek in optimizing alarm design.
9 Limitations
There are limitations to this study. First, undergraduate students were participants, who are not the end-users of ICU alarms, but are proper for this feasibility study as they serve as a novice population which brings workload perspectives from those outside the medical field. Also, this was conducted with cognitive tasks that have been previously used in the literature to simulate the ICU cognitive load, though it is not a direct comparison to the real ICU environment. Finally, this feasibility study cannot comment on is the performance of this alarm device longitudinally. This will be a key aspect in future research, as the nature of alarm fatigue does not develop within a short laboratory time, but rather due to the lengthy exposure to the alarms. This is a common limitation of alarm research; though there must be proof of concept data to support clinical integration, prior to clinical testing, which this study serves well as.
10 Conclusion
Overall, this investigation demonstrated an improvement in workload with a multisensory alarm system, without detriments in participant alarm identification performance. Our results suggest that with the proper implementation and integration within the current medical alarm environment, medical personnel may experience a benefit to the current alarm environment in the form of an environment relying less heavily on auditory senses in addition to increased sensory integration such that there may be a reduction in the incidence of alarm fatigue.
Code availability
Any and all data including supplemental data is available via request of the author.
References
Schlesinger JJ, Miller SHB, Nash K, et al. Acoustic features of auditory medical alarms—An experimental study of alarm volume. J Acoust Soc Am. 2018;143(6):3688. https://doi.org/10.1121/1.5043396.
Bitan Y, Meyer J, Shinar D, Zmora E. Nurses’ reactions to alarms in a neonatal intensive care unit. Cogn Technol Work. 2004;6(4):239–46. https://doi.org/10.1007/s10111-004-0162-2.
Imhoff M, Kuhls S. Alarm algorithms in critical care monitoring. Anesth Analg. 2006;102(5):1525–37. https://doi.org/10.1213/01.ANE.0000204385.01983.61.
Talley LB, Hooper J, Jacobs B, et al. Cardiopulmonary monitors and clinically significant events in critically Ill Children. Biomed Instrum Technol. 2011;45(s1):38–45. https://doi.org/10.2345/0899-8205-45.s1.38.
Stevenson RA, Schlesinger JJ, Wallace MT. Effects of divided attention and operating room noise on perception of pulse oximeter pitch changes. Anesthesiology. 2013;118(2):376–81. https://doi.org/10.1097/ALN.0b013e31827d417b.
The Joint Commission. National patient safety goals. Published online 2022.
Darbyshire JL, Müller-Trapet M, Cheer J, Fazi FM, Young JD. Mapping sources of noise in an intensive care unit. Anaesthesia. 2019;74(8):1018–25. https://doi.org/10.1111/anae.14690.
Graham KC, Cvach M. Monitor alarm fatigue: standardizing use of physiological monitors and decreasing nuisance alarms. Am J Crit Care. 2010;19(1):28–34. https://doi.org/10.4037/ajcc2010651.
Wee AN, Sanderson PM. Are Melodic Medical Equipment Alarms easily learned? Anesth Analg. 2008;106(2):501–8. https://doi.org/10.1213/01.ane.0000286148.58823.6c.
Burdick KJ, Callahan CJ. Sleeping soundlessly in the Intensive Care Unit. Multimodal Technol Interact. 2020;4(1):6. https://doi.org/10.3390/mti4010006.
Burdick KJ, Jorgensen SK, Combs TN, Holmberg MO, Kultgen SP, Schlesinger JJ. SAVIOR ICU: sonification and vibrotactile interface for the operating room and intensive care unit. J Clin Monit Comput. 2020;34(4):787–96. https://doi.org/10.1007/s10877-019-00381-1.
Torabizadeh C, Yousefinya A, Zand F, Rakhshan M, Fararooei M. A nurses’ alarm fatigue questionnaire: development and psychometric properties. J Clin Monit Comput. 2017;31(6):1305–12. https://doi.org/10.1007/s10877-016-9958-x.
Fleishman S, Hess A, Sloan L, Schlesinger JJ, Shive J. Detecting Abnormalities on Displays of Patient Information. In:; 2021:287–300. doi:https://doi.org/10.1007/978-3-030-74611-7_40
Spence C, Ho C. Tactile and multisensory spatial warning signals for drivers. IEEE Trans Haptics. 2008;1(2):121–9. https://doi.org/10.1109/TOH.2008.14.
Ho C, Gray R, Spence C. Reorienting driver attention with dynamic tactile cues. IEEE Trans Haptics. 2014;7(1):86–94. https://doi.org/10.1109/TOH.2013.62.
Cao CGL, Zhou M, Jones DB, Schwaitzberg SD. Can Surgeons think and operate with Haptics at the same time? J Gastrointest Surg. 2007;11(11):1564–9. https://doi.org/10.1007/s11605-007-0279-8.
Katzman N, Gellert M, Schlesinger JJ, Oron-Gilad T, Cooperstock JR, Bitan Y. Evaluation of tactile cues for simulated patients’ status under high and low workload. Proceedings of the Human Factors and Ergonomics Society Annual Meeting. 2019;63(1):658–662. doi:https://doi.org/10.1177/1071181319631285
International Electrotechnical Commission. : Geneva Switzerland. IEC 60601-1-8: 2006 + AMD1: 2012 + AMD2. 2020 CSV Consolidated Version. Published online 2020.
Kirchner WK. Age differences in short-term retention of rapidly changing information. J Exp Psychol. 1958;55(4):352–8. https://doi.org/10.1037/H0043688.
Coulacoglou C, Saklofske DH. Executive Function, Theory of Mind, and Adaptive Behavior. Psychometrics and Psychological Assessment.Elsevier; 2017:pp. 91–130. doi:https://doi.org/10.1016/B978-0-12-802219-1.00005-5
Said S, Gozdzik M, Roche TR, et al. Validation of the Raw National Aeronautics and Space Administration Task load index (NASA-TLX) Questionnaire to assess Perceived workload in patient monitoring tasks: pooled analysis study using mixed models. J Med Internet Res. 2020;22(9). https://doi.org/10.2196/19472.
Fetsch CR, DeAngelis GC, Angelaki DE. Bridging the gap between theories of sensory cue integration and the physiology of multisensory neurons. Nat Rev Neurosci. 2013;14(6):429–42. https://doi.org/10.1038/nrn3503.
Stein BE, Stanford TR, Rowland BA. Development of multisensory integration from the perspective of the individual neuron. Nat Rev Neurosci. 2014;15(8):520–35. https://doi.org/10.1038/nrn3742.
Deb S, Claudio D. Alarm fatigue and its influence on staff performance. IIE Trans Healthc Syst Eng. 2015;5(3):183–96. https://doi.org/10.1080/19488300.2015.1062065.
Sanderson PM, Wee A, Lacherez P. Learnability and discriminability of melodic medical equipment alarms*. Anaesthesia. 2006;61(2):142–7. https://doi.org/10.1111/j.1365-2044.2005.04502.x.
Edworthy JR, Parker CJ, Martin E. Discriminating between simultaneous audible alarms is easier with auditory icons. Appl Ergon. 2022;99:103609. https://doi.org/10.1016/j.apergo.2021.103609.
Acknowledgements
We would like to thank Vanderbilt Medical Center’s Department of Anesthesiology and The Vanderbilt University’s Neuroscience Program. We would also like to thank Tamara Naidoo for aiding in the scheduling of the participant run times during the data collection phase of this experiment.
Funding
This work was supported by Office of Naval Research Grant N00014-22-1-2184.
The authors declare that no other funds, grants, or any other support were received during this preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Development of the Visual Studio Code for the experimental tool was performed by May Gellert. Material preparation and data collection were performed by Derek Rios. The data analysis detailed in the methods and results sections were completed by Nuphar Katzman (all, except NASA-TLX), Jessica Klein, and Derek Rios (NASA-TLX analysis). The first draft of the manuscript was written by Derek Rios, and Kendall Burdick was the primary commenter and editor on previous versions of the manuscript leading up to the finalized work. Dr. Joseph Schlesinger and Dr. Yuval Bitan served as this team’s principal investigators.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was performed in line with the principles of Vanderbilt University. Additionally, the approval of the Vanderbilt Human Research Protection Program – HPPP (Supporting work of the Institutional Review Board) was granted on February 2nd, 2020. Informed consent was obtained and recorded from all participants in this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rios, D., Katzman, N., Burdick, K.J. et al. Multisensory alarm to benefit alarm identification and decrease workload: a feasibility study. J Clin Monit Comput 37, 1051–1059 (2023). https://doi.org/10.1007/s10877-023-01014-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-023-01014-4