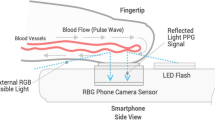
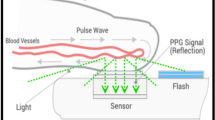
Abstract
We compared blood pressure (BP) values obtained with a new optical smartphone application (OptiBP™) with BP values obtained using a non-invasive automatic oscillometric brachial cuff (reference method) during the first 2 h of surveillance in a post-anesthesia care unit in patients after non-cardiac surgery. Three simultaneous BP measurements of both methods were recorded every 30 min over a 2-h period. The agreement between measurements was investigated using Bland–Altman and error grid analyses. We also evaluated the performance of the OptiBP™ using ISO81060–2:2018 standards which requires the mean of the differences ± standard deviation (SD) between both methods to be less than 5 mmHg ± 8 mmHg. Of 120 patients enrolled, 101 patients were included in the statistical analysis. The Bland–Altman analysis demonstrated a mean of the differences ± SD between the test and reference methods of + 1 mmHg ± 7 mmHg for mean arterial pressure (MAP), + 2 mmHg ± 11 mmHg for systolic arterial pressure (SAP), and + 1 mmHg ± 8 mmHg for diastolic arterial pressure (DAP). Error grid analysis showed that the proportions of measurement pairs in risk zones A to E were 90.3% (no risk), 9.7% (low risk), 0% (moderate risk), 0% (significant risk), 0% (dangerous risk) for MAP and 89.9%, 9.1%, 1%, 0%, 0% for SAP. We observed a good agreement between BP values obtained by the OptiBP™ system and BP values obtained with the reference method. The OptiBP™ system fulfilled the AAMI validation requirements for MAP and DAP and error grid analysis indicated that the vast majority of measurement pairs (≥ 99%) were in risk zones A and B.
Trial Registration ClinicalTrials.gov Identifier: NCT04262323.






Similar content being viewed by others
References
Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16:223–37.
Floras JS, Jones JV, Hassan MO, Osikowska B, Sever PS, Sleight P. Cuff and ambulatory blood pressure in subjects with essential hypertension. Lancet. 1981;2:107–9.
Patel AA. Developing and evaluating mHealth solutions for chronic disease prevention in primary care. Circulation. 2019;139:392–4.
Michard F. Smartphones and e-tablets in perioperative medicine. Korean J Anesthesiol. 2017;70:493–9.
Michard F, Barrachina B, Schoettker P. Is your smartphone the future of physiologic monitoring? Intensive Care Med. 2019;45:869–71.
Michard F. Toward smart monitoring with phones, watches, and wearable sensors. Anesthesiol Clin. 2021;39:555–64.
Hoppe P, Gleibs F, Briesenick L, Joosten A, Saugel B. Estimation of pulse pressure variation and cardiac output in patients having major abdominal surgery: a comparison between a mobile application for snapshot pulse wave analysis and invasive pulse wave analysis. J Clin Monit Comput 2021;35(5):1203–9
Joosten A, Boudart C, Vincent JL, et al. Ability of a new smartphone pulse pressure variation and cardiac output application to predict fluid responsiveness in patients undergoing cardiac surgery. Anesth Analg. 2019;128:1145–51.
Desebbe O, Vincent JL, Saugel B, Rinehart J, Joosten A. Pulse pressure variation using a novel smartphone application (Capstesia) versus invasive pulse contour analysis in patients undergoing cardiac surgery: a secondary analysis focusing on clinical decision making. J Clin Monit Comput. 2020;34:379–80.
Joosten A, Jacobs A, Desebbe O, et al. Monitoring of pulse pressure variation using a new smartphone application (Capstesia) versus stroke volume variation using an uncalibrated pulse wave analysis monitor: a clinical decision making study during major abdominal surgery. J Clin Monit Comput. 2019;33:787–93.
Desebbe O, Joosten A, Suehiro K, et al. A novel mobile phone application for pulse pressure variation monitoring based on feature extraction technology: a method comparison study in a simulated environment. Anesth Analg. 2016;123:105–13.
Ghamri Y, Proença M, Hofmann G, et al. Automated pulse oximeter waveform analysis to track changes in blood pressure during anesthesia induction: a proof-of-concept study. Anesth Analg. 2020;130:1222–33.
Jorge J, Proenca M, Aguet C, et al. Machine learning approaches for improved continuous, non-occlusive arterial pressure monitoring using photoplethysmography. Annu Int Conf IEEE Eng Med Biol Soc. 2020;2020:910–3.
Schoettker P, Degott J, Hofmann G, et al. Blood pressure measurements with the OptiBP smartphone app validated against reference auscultatory measurements. Sci Rep. 2020;10:17827.
Degott J, Ghajarzadeh-Wurzner A, Hofmann G, et al. Smartphone based blood pressure measurement: accuracy of the OptiBP mobile application according to the AAMI/ESH/ISO universal validation protocol. Blood Press Monit 2021
Desebbe O, Tighenifi A, Jacobs A, et al. Evaluation of a novel mobile phone application for blood pressure monitoring: a proof of concept study. J Clin Monit Comput 2021
Ramsey M 3rd. Blood pressure monitoring: automated oscillometric devices. J Clin Monit. 1991;7:56–67.
Bland JM, Altman DG. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat. 2007;17:571–82.
Stergiou GS, Palatini P, Asmar R, et al. Recommendations and Practical Guidance for performing and reporting validation studies according to the Universal Standard for the validation of blood pressure measuring devices by the Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO). J Hypertens. 2019;37:459–66.
Grothe O, Kaplan A, Kouz K, Saugel B. Computer program for error grid analysis in arterial blood pressure method comparison studies. Anesth Analg. 2020;130:e71–4.
Saugel B, Grothe O, Nicklas JY. Error grid analysis for arterial pressure method comparison studies. Anesth Analg. 2018;126:1177–85.
Kottner J, Audigé L, Brorson S, et al. Guidelines for Reporting Reliability and Agreement Studies (GRRAS) were proposed. J Clin Epidemiol. 2011;64:96–106.
Cecconi M, Dawson D, Grounds RM, Rhodes A. Lithium dilution cardiac output measurement in the critically ill patient: determination of precision of the technique. Intensive Care Med. 2009;35:498–504.
Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36:1953–2041.
Acknowledgements
All the clinicians and nurses from the emergency department.
Funding
This work was supported by the Department of Anesthesiology, Erasme Hospital, Brussels.
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript. OD: designed the study, analyzed the data and edited the final manuscript. MEH: collected and analyzed the data and edited the final manuscript. KK: collected the data and edited the final manuscript. BA: analyzed the data and edited the final manuscript. LK: analyzed the data and edited the final manuscript. DC: analyzed the data and edited the final manuscript. JFK: analyzed the data and edited the final manuscript. JD: analyzed the data and edited the final manuscript. PS: analyzed the data and edited the final manuscript. FM: analyzed the data and edited the final manuscript. BS: analyzed the data and edited the final manuscript. JLV: analyzed the data and edited the final manuscript. AJ: designed the study, analyzed the data and drafted the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
OD is consultant for Medtronic (Trévoux, FRANCE) and and Livanova (Châtillon, France). JFK is working for Biospectal SA, Lausanne, Switzerland. PS is an advisor of Biospectal SA, Lausanne, Switzerland. AJ is a consultant for Edwards Lifesciences (Irvine, California, USA). The other authors have no conflicts of interest to declare.
Ethical approval
The present study was approved by the ethics committee of Erasme Hospital on October 20th, 2020 under the reference A2020/199 and registered in Clinical Trial.gov on February 10th, 2020 under the reference NCT04262323 (Principal Investigator: Alexandre Joosten).
Consent to participate
Written informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1: Boxplots of mean of the differences at each time point
Appendix 1: Boxplots of mean of the differences at each time point

Rights and permissions
About this article
Cite this article
Desebbe, O., El Hilali, M., Kouz, K. et al. Evaluation of a new smartphone optical blood pressure application (OptiBP™) in the post-anesthesia care unit: a method comparison study against the non-invasive automatic oscillometric brachial cuff as the reference method. J Clin Monit Comput 36, 1525–1533 (2022). https://doi.org/10.1007/s10877-021-00795-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-021-00795-w