Abstract
Recently, we developed a novel endotracheal catheter with functional cuff (ECFC). Using such an ECFC and a regular ICU ventilator, we were able to generate clinically relevant tidal volume in a lung model and adult human sized animal. This ECFC allows co-axial ventilation without using a jet ventilator. The aim of this study was to determine if ECFC also could generate clinically relevant positive end expiratory pressure (PEEP). The experiment was conducted on a model lung and artificial trachea. Lung model respiratory mechanics were set to simulate those of an adult human being. The tip of the distal end of ECFC 14 or 19 Fr catheter was positioned in the artificial trachea 3 cm above the carina. The proximal end of ECFC was connected to an ordinary ICU ventilator. With 14 Fr catheter at respiratory rate 10 bpm, PEEP 0, 2.9, 8.2, 12.9 cmH2O was generated at preset PEEP 0, 5, 10, 15 cmH2O respectively and tidal volume was up to 393.4 ml. With 19 Fr catheter, PEEP was 0, 2.8, 7.6, 12.3 cmH2O, at preset PEEP 0, 5, 10, 15 cmH2O respectively and the tidal volume was up to 667.3 ml. With 14 Fr catheter at respiratory rate 20 bpm, PEEP was 0, 3.9, 9.6, 14.6 cmH2O at preset PEEP 0, 5, 10, 15 cmH2O respectively and tidal volume was up to 188.8 ml. With 19 Fr catheter, PEEP was 0, 3.6, 8.9, 13 cmH2O, at preset PEEP 0, 5, 10, 15 cmH2O respectively and tidal volume was up to 345.3 ml. ECFC enables clinicians to generate not only adequate tidal volume but also clinically relevant PEEP via co-axial ventilation using an ordinary ICU ventilator.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
1 Introduction
Airway exchange catheters (AEC) have been widely used to facilitate extubation and re-intubation with a known difficult airway [1,2,3,4,5,6]. The long hollow thin tube is inserted through an endotracheal tube (ETT) before extubation to provide continuous airway access and left in place in the trachea as a guide after removal of ETT [7]. In critical care settings, the primary use of AEC has been as a tube exchanger. When attached to jet ventilation, continuous oxygen can be provided through the distal end of the catheter and its side ports. Thus, AECs can be used to ventilate patients affording the physician time to consider alternative airway management approaches. This practice has minimized the occurrence of complications associated with extubation, especially in at-risk patients with airway obstruction, respiratory pathologies or undergoing maxillofacial or neck surgery [8,9,10]. A prospective study of 354 patients with difficult airways over a 9-year period confirmed the efficacy and relative safety of AECs [10]. The complications, including hypoxia, hypotension, bradycardia, esophageal intubation, and use of accessory airway adjuncts were decreased when using an AEC to reintubate [9, 11, 12]. In patients with difficult airways whose tracheas were re-intubated with or without an AEC, Mort demonstrated that the AEC provides significant improvement in the first attempt success rate, the time to complete re-intubation, a reduction in bradycardia and severe hypoxia [10].
Despite these benefits, severe risks/complications associated with AECs still occur [13,14,15,16]. Barotrauma resulting in pneumothorax has been a major concern when using jet ventilation through AECs [8, 17, 20]. It has been reported that barotrauma, cardiac arrest, pneumothorax and death are all associated with jet ventilation via AEC [8, 14, 18, 19] and there is a need for excessive airway driving pressure with jet ventilation [21]. Therefore, minimizing airway pressure and providing a long expiratory time has been proposed to decrease the incidence of complicating pneumothorax [18, 21]. Furthermore, jet ventilation may not be readily available in some emergency cases [22, 23]. These disadvantages have led to questions regarding the utility of jet ventilation via an AEC.
Recently, we developed an endotracheal catheter with a functional cuff (ECFC) by modifying an AEC and demonstrated that ECFC can generate clinically relevant tidal volumes using a regular ICU ventilator instead of a jet ventilator in a lung model study [24] as well as in an adult human sized animal [25]. The aim of the current study was to determine if ECFC using an ICU ventilator produces clinically relevant positive end expiratory pressure (PEEP).
2 Methods
2.1 Endotracheal catheter equipped with a functional cuff (ECFC)
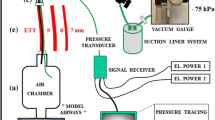
A Cook Airway Exchange Catheter (3.0 mm I.D-14Fr or 3.6 mm-19Fr, Cook Critical Care, Bloomington, IN, USA) was used to create an ECFC as described in our previous study [21, 25]. Briefly, a 5 cm length, diameter 27 mm taper PU cuff was added at the distal end of the airway exchange catheter, creating an endotracheal catheter with a functional cuff (Fig. 1). Two side ports (one on each side) covered by PU cuff were enlarged to 10 mm × 2.5 mm for 19Fr catheter and 10 mm × 2 mm for 14Fr catheter. The total area of the two orifices of the cuff is 50 mm2 on 19Fr catheter and 40 mm2 on 14Fr catheter. A resistor was added at the tip of the catheter in order to create a gradient between the intra-cuff pressure and airway pressure distal to the tip of ECFC. The inner diameter of the resistor is 2.1 mm for the 14Fr and 2.3 mm for the 19Fr catheter. A tracheal model with an internal diameter of 22 mm was used in this study. At full insufflation of the cuff, the diameter of the cuff is 27 mm, 5 mm greater than that of the lumen of the trachea. The operation principle is described as follows: inspiratory flow creates a pressure gradient across the cuff due to the presence of the resistor and enables the cuff to be inflated only during the inspiratory phase. Upon the beginning of exhalation pressure in the ECFC drops to baseline (PEEP). The recoil force of the respiratory system and pressure in the cuff deflates the cuff and exhalation occurs not via the lumen of ECFC but around it. Ventilation was provided with an ordinary ICU ventilator using pressure control with peak inspiratory pressure (PIP) 25, 50, and 70 cmH2O and preset PEEP 0, 5, 10 and 15 cmH2O. The distal end of ECFC was placed in the trachea 3 cm above the carina and the proximal end attached to the ventilator.
2.2 Study procedure
A flow/pressure sensor (NICO Cardiopulmonary Management System, Model 7300, Respironics Corp., Murrysville, PA, USA) was placed on the tracheal model distal to the tip of catheter. It was automatically calibrated during data collection and data collecting was at a sampling rate of 100 Hz. The ICU ventilation (Evita 4, Dräger, Lübeck, Germany) was set in pressure-controlled mode with respiratory rate of 10 or 20 breaths per minute; inspiratory to expiratory (I:E) ratios of 1:2 and 1:1 at each ventilatory setting for 30 s. Compliance of the lung model was 20 ml cmH2O−1 (low), resistance 5 cmH2O l−1 s−1 and 50 ml cmH2O−1 (normal), resistance 2 cmH2O l−1 s−1.
After measurements with 14 Fr ECFC were completed, the 14 Fr ECFC was replaced with the19 Fr ECFC and measurements were repeated. At each ventilatory setting, five breaths were collected. Repeated tests showed that a steady state of PEEP was achieved after the first breath. The last three breaths of 3rd, 4th, and 5th were used for calculation of PEEP and VT. The mean value of the three breaths was treated as a single data points and the measurement was repeated three times on different days. The VT and PEEP values obtained from the three experiments were used to calculate the mean and standard deviation.
2.3 Statistical analysis
Data were presented as mean ± standard deviation. A VT greater than 150 ml was considered an effective VT. The Independent-Sample T test was performed to identify the significance of the difference in PEEP and VT at variable ventilatory settings. Differences were considered statistically significant when p < 0.01. Statistical analysis was done with a statistical software package (PASW Statistic 18; SPSS; Chicago, IL).
3 Results
3.1 PEEP achieved using ECFC
The mean PEEP levels generated with ECFC are presented in Fig. 2 and Tables 1 and 2. At ventilation rate of 10 bpm, mean PEEP with the 14 Fr ECFC were 0, 3.3, 8.8, and 13.6 cmH2O at preset PEEP 0, 5, 10, and 15 cmH2O with normal respiratory compliance (14FrNC) and 0, 2.5, 7.6, and 12.2 cmH2O at the preset PEEP 0, 5, 10, and 15 cmH2O with low compliance (14FrLC) respectively. With the 19 Fr catheter mean PEEP were 0, 3.0, 8.2, and 12.9 cmH2O at preset PEEP 0, 5, 10, and 15 cmH2O with normal compliance (19FrNC) and 0, 2.7, 7.1, and 11.7 cmH2O at preset PEEP 0, 5, 10, and 15 cmH2O with low compliance (19FrLC) respectively.
Actual PEEP and tidal volumes achieved with 14 or 19Fr ECFC at variable preset PEEP, different I:E ratios and respiratory rate of 10 bpm. Panels on the left side illustrate the actual PEEP value and panels on the right present the corresponding tidal volumes obtained with 14 or 19Fr endotracheal catheter with a functional cuff (ECFC) at low compliance (LC) or normal compliance (NC). 14FrLC or 19FrLC, 14 or 19Fr ECFC at low compliance; 14FrNC or 19FrNC, 14 or 19Fr ECFC at normal compliance; Peak inspiratory pressure (PIP) was set at 25, 50 or 70 cmH2O, and positive end expiratory pressure (PEEP) was at 0, 5, 10, 15 cmH2O. I:E ratio, inspiratory to expiratory ratio; I:E ratio was 1:2 or 1:1
At respiratory rate of 20 bpm, PEEP were 0, 4.5, 10.1, and 14.9 cmH2O at preset PEEP 0, 5, 10, and 15 cmH2O with 14FrNC and 0, 3.3, 9.1, 14.3 and 12.2 cmH2O at preset PEEP 0, 5, 10, and 15 cmH2O with 14FrLC respectively. PEEP of 0, 4.3, 10.3 and 13.7 cmH2O were measured at preset PEEP 0, 5, 10, and 15 cmH2O with 19FrNC and 0, 2.9, 7.4, and 12.3 cmH2O at preset PEEP 0, 5, 10, and 15 cmH2O with 19FrLC respectively.
The difference between preset PEEP and actual PEEP with 19Fr ECFC was significantly greater than that with 14Fr ECFC (p < 0.05, Fig. 3). Compared with normal compliance, greater PEEP was generated with low compliance but the differences were not significant (p > 0.05, Fig. 3).
Differences between preset PEEP and Actual PEEP with 14Fr and 19 Fr ECFC at variable respiratory rate and normal or low compliance. a Differences between preset PEEP and Actual PEEP with 14Fr and 19Fr endotracheal catheter with a functional cuff (ECFC) at respiratory rate of 10 bpm; b differences between preset PEEP and Actual PEEP with 14Fr and 19Fr ECFC at respiratory rate of 20 bpm. 14FrLC or 19FrLC, 14 or 19Fr ECFC at low compliance; 14FrNC or 19FrNC, 14 or 19Fr ECFC at normal compliance; Peak inspiratory pressure (PIP) was set at 25, 50 or 70 cmH2O, and positive end expiratory pressure (PEEP) was at 5, 10, 15 cmH2O. I:E ratio was 1:2 or 1:1
3.2 Effects of I:E ratios and inspiratory time on PEEP
At the same respiratory rate, there was a trend of greater PEEP with longer inspiratory time but differences were not significant (p > 0.05) (Figs. 2, 4; Tables 1, 2).
Actual PEEP and tidal volumes achieved with 14 or 19Fr ECFC at variable preset PEEP, different I:E ratios and respiratory rate of 20 bpm. Panels on the left side illustrate the actual PEEP value and panels on the right present the corresponding tidal volumes obtained with 14 or 19Fr endotracheal catheter with a functional cuff (ECFC) at low compliance (LC) or normal compliance (NC). 14FrLC or 19FrLC, 14 or 19Fr ECFC at low compliance; 14FrNC or 19FrNC, 14 or 19Fr ECFC at normal compliance; Peak inspiratory pressure (PIP) was set at 25, 50 or 70 cmH2O, and positive end expiratory pressure (PEEP) was at 0, 5, 10, 15 cmH2O. I:E ratio, inspiratory to expiratory ratio; I:E ratio was 1:2 or 1:1
3.2.1 VT achieved using ECFC
The mean VT are presented in Figs. 2, 4 and Tables 3, 4. VT was 0–188.8 ml with ECFC 14Fr and 96–667 ml with ECFC 19Fr when respiratory rate was at 20 bpm. At ventilation rate of 10 bpm, Vt was 0–393 ml with 14Fr and 45.6–345.3 ml with 19Fr (Figs. 2, 4; Tables 3, 4).
3.2.2 VT achieved with different I:E ratios
Greater VT was generated with longer inspiratory time among all groups (p < 0.01) (Figs. 2, 4; Tables 3, 4).
4 Discussion
The major findings of this study are: (i) ICU ventilator and ECFC were able to generate clinically relevant PEEP, (ii) the actual PEEP produced by ECFC approximates the preset PEEP, (iii) a minimal inspiratory time of 1 s was required to produce PEEP and (iv) respiratory compliance does not significantly affect PEEP generation. To the best of our knowledge, this is the first study to evaluate efficacy of PEEP generation using ECFC and an ICU ventilator. Our results indicate that ECFC enables clinicians to use ordinary ICU ventilators to achieve effective ventilation and obtain clinically relevant PEEP levels.
In our previous study, adequate tidal volume was produced using a regular ICU ventilator and ECFC [24, 25]. This current study demonstrates that the ECFC can generate clinically relevant PEEP levels. We believe that the PEEP was created as follows (Fig. 4): during the first inspiration, the inflow creates a pressure gradient at the tip of the ECFC due to the presence of a resistor. On the distal side of ECFC, side ports covered by PU cuff is 10 × 2 mm for 14Fr catheter and 10 × 2.5 mm for 19Fr catheter. The two orifices of the cuff are 10 × 2 mm, 40 mm2 on 14Fr catheter and 10 × 2.5 mm, 50 mm2 on 19Fr catheter. The inner diameter of the resister is 2.1 mm and 2.3 mm for 14Fr and 19Fr catheter respectively. Its cross-section area is 3.5 mm2 and 4.2 mm2 which is at least 10 times smaller than that of the total cross section area of the inflating orifice. Therefore, the differential resistance between the inflating orifices and the resister is large. The intra-cuff pressure is equal to the sum of intra-tracheal pressure distal to the tip of ECFC and the pressure gradient across the resistor. Because the intra-cuff pressure is greater than the intra-tracheal pressure distal to the tip of the ECFC, and at full insufflation, the diameter of the PU cuff is greater than that of the lumen of the trachea, the cuff inflates and seals the tracheal lumen. Therefore, there is no or minimal back flow at and around the cuff. Intra-cuff pressure continues to rise until reaching the preset PIP which is the sum of the intra-tracheal pressure and the pressure gradient across the resistor. When the preset inspiratory time is reached, in-flow ceases and the ventilator pressure goes to baseline (set PEEP). Intra-cuff pressure is higher than the intraluminal ECFC pressure and the cuff collapses and expiration begins. Expiration occurs around, but not through the catheter. However, the ventilator maintains intra-cuff pressure at the preset PEEP. The ventilator is set in pressure control and after the appropriate expiratory time the next inspiration is provided. The ventilator continually maintains flow to establish PEEP insuring the intra-cuff pressure is always approximately equal to that in the distal tracheal lumen pressure and insuring the applied PEEP is approximately equal to the set PEEP. In this bench study, oxygen flows through ECFC, which is similar but not identical to jet ventilation. This is because ECFC has inflated and sealed the trachea before inspiration occurrence and maintains inflated during inspiration. Therefore, ECFC likely generates a jet flow with velocity similar to that of jet ventilation but no entraining gas involved.
Through the entire experiment, we observed that when respiratory rate was at 10 breaths per minute, the preset PEEP was never reached and the actually achieved PEEP was always 1–4 cmH2O lower than the preset PEEP as shown in Fig. 2a, c, e, g. This is likely due to the pressure gradient across the resistor and/or the intrinsic re-coil force of the cuff itself. If this notion is correct, the preset PEEP should be the sum of the actual PEEP and the pressure gradient at the resistor. However, we did not measure the pressure gradient across the cuff. Because the pressure gradient is flow rate dependent due to presence of the resistor, the greater the resistance of the resistor, the larger the difference between the preset PEEP and the actual PEEP. This point can be clearly seen by comparing the differences between the preset PEEP and actual PEEP between the two sizes of catheters. The 19 Fr catheter produces larger tidal volume than 14 Fr at each ventilatory setting. The total resistance of the 14 Fr catheter is greater than that of 19Fr catheter and the pressure gradient across the resistor must be the same at a given tidal volume. Reduction in resistance of the resistor should lead to a decrease in the difference of preset PEEP and actual PEEP (Fig. 3). But further study is needed to confirm this notion and verify if the reduction in resistance of the resistor affects greater generation of PEEP. We also observed that at ventilation rate 20 bpm, the actual PEEP was even greater than the preset PEEP (I:E = 1:1). This implies the shorter expiration time and higher respiratory compliance might cause air trapping. We did not observe such phenomena at low compliance. This is likely due to the greater re-coil force at low compliance than that at high compliance. It takes less time to expel the air out and the time of expiration is long enough to allow complete expiration. Therefore, there was no air trapping at low compliance.
Our current study did not evaluate the ECFC in patients, and as a result we can only speculate its effects on patient outcome. However, there are two potential advantages of this ventilation method. (1) It reduces dead space ventilation (estimated about 50% reduction), due to co-axial ventilation, as the interface of the fresh gas and CO2 containing gas is moved from the Y-piece of the breathing circuit to near the carina of the airway. This feature allows clinicians to significantly reduce tidal volume at constant PIP. Therefore, it can provide “protective ventilation” [25]. (2) There is no native airway bypassed during expiration via co-axial ventilation and expiration occurs through the entire native airway. In contrast with a regular endotracheal tube, this feature should facilitate secretion removal from trachea. A regular endotracheal tube creates a segment of airway above the cuff without air flow. Accumulation of secretions above a normal cuff serves as a culture for bacteria growth and increases the probability of ventilator associated pneumonia [26].
There are a few potential implications of the device. (1) It can serve as the regular airway exchange catheter and provide mechanical ventilation. Ventilation can be provided with a regular ICU or operating room ventilator. (2) The ECFC and a regular ventilator may be able to replace trachea catheter puncture and jet ventilator for laryngeal surgeries. Surgeons prefer the use of endotracheal catheters together with jet ventilator [27]. However, jet ventilator has several disadvantages as mentioned in the introduction. ECFC can easily meet all of the functional requirements of laryngeal surgeries but not requiring jet ventilation. (3) Most electrophysiology laboratories employ jet ventilation for pulmonary vein isolation [28, 29], due to the requirement of tidal volume less than 200 ml in order to minimize the motion generated by breathing or ventilation. Currently, this task is accomplished with jet ventilators. ECFC can easily produce tidal volume less than 200 ml without using jet ventilation. (4) Surgeons often use jet ventilation and a small catheter to perform trachea resections and anastomosis. ECFC can replace the catheter and jet ventilator. And (5) ECFC potentially can be used during general anaesthesia and replace a regular ETT or LMA. However, further study is needed to test this notion and aspiration is a major concern since ECFC, like most forms of jet ventilation, does not provide a secured airway.
There are a few limitations to this study. Firstly, the study was not conducted on humans but rather conducted using a tracheal/lung model to simulate a normal adult or ARDS scenario. Second, the texture of human trachea wall is different from that of the artificial trachea we used in this study and such differences may affect the seal of the cuff and PEEP generation. Therefore, large animal studies should be done before testing on humans. Thirdly, we did not test catheters smaller than 14 Fr and larger than 19Fr. Airflow dynamic will be different if the inner diameter of the catheter changes. Forth, the resistance of the resistor was arbitrarily chosen and we did not test variable resistors. The change in the resistance of the resistor may alter the creation and/or maintenance of PEEP. Finally, we did not compare different sizes of the cuffs made of different materials. Optimizing cuff design and cuff material is required to maximize efficacy of ventilation and PEEP.
In conclusion, the use of a regular ICU ventilator and ECFC allows for the generation of clinically relevant PEEP and tidal volumes. The level of applied PEEP approximates preset PEEP levels. Further study in large animal and human is needed to validate our observation and optimize the design of ECFC.
Abbreviations
- AEC:
-
Airway exchange catheter
- ECFC:
-
Endotracheal catheter with functional cuff
- PEEP:
-
Positive end expiratory pressure
- ETT:
-
Endotracheal tube
- ICU ventilator:
-
Intensive care unit ventilator
- NICO:
-
Non-invasive cardiac output monitor
- bpm:
-
Breath per minute
References
Moyers G, McDougle L. Use of the COOK airway exchange catheter in “bridging” the potentially difficult extubation: a case report. AANA J. 2002;70:275–8.
Bedger RC, Chang JL. A jet-stylet endotracheal catheter for difficult airway management. Anesthesiology 1987;66:221–3.
Gitman M, Mueller M. The use of an airway exchange catheter during a difficult tracheostomy. J Neurosurg Anesthesiol. 2012;24:78–9.
Mort TC, Braffett BH. Conventional versus video laryngoscopy for tracheal tube exchange: glottic visualization, success rates, complications, and rescue alternatives in the high-risk difficult airway patient. Anesth Analg. 2015;121:440–8.
Candido KD, Saatee S, Appavu SK, Khorasani A. Revisiting the ASA guidelines for management of a difficult airway. Anesthesiology 2000;93:295–8.
Gamez R, Slinger P. A simulator study of tube exchange with three different designs of double-lumen tubes. Anesth Analg. 2014;119:449–53.
Popat M, Mitchell V, Dravid R, Patel A, Swampillai C, Higgs A. Difficult Airway Society Guidelines for the management of tracheal extubation. Anaesthesia 2012;67:318–40.
Cooper RM, Cohen DR. The use of an endotracheal ventilation catheter for jet ventilation during a difficult intubation. Can J Anaesth. 1994;41:1196–9.
Dosemeci L, Yilmaz M, Yegin A, Cengiz M, Ramazangolu A. The routine use of a pediatric airway exchange catheter after extubation of adult patients who have undergone maxillofacial or major neck surgery: a clinical observational study. Crit Care. 2004;8:385–90.
Mort TC. Continuous airway access for the difficult extubation: the efficacy of the airway exchange catheter. Anesth Analg. 2007;105:1357–62.
Loudermilk EP, Hartmannsgruber M, Stoltzfus DP, Langevin PB. A prospective study of the safety of tracheal extubation using a pediatric airway exchange catheter for patients with a known difficult airway. Chest 1997;111:1660–5.
Mort TC. Tracheal tube exchange: feasibility of continuous glottic viewing with advanced laryngoscopy assistance. Anesth Analg. 2009;108:1228–31.
Cooper RM. The use of an endotracheal ventilation catheter in the management of difficult extubations. Can J Anaesth. 1996;43:90–3.
Rashid AM, Williams C, Noble J, Rashid OM, Takabe K, Anand RJ. Pneumothorax, an underappreciated complication with an airway exchange catheter. J Thorac Dis. 2012;4:659–62.
Nunn C, Uffman J, Bhananker SM. Bilateral tension pneumothoraces following jet ventilation via an airway exchange catheter. J Anesth. 2007;21:76–9.
Fetterman D, Dubovoy A, Reay M. Unforeseen esophageal misplacement of airway exchange catheter leading to gastric perforation. Anesthesiology 2006;104:1111–2.
Benumof JL. Airway exchange catheters: simple concept, potentially great danger. Editorial views. Anesthesiology 1999;91:342–4.
Duggan LV, Law JA, Murphy MF. Brief review: supplementing oxygen through an airway exchange catheter: efficacy, complications, and recommendations. Can J Anaesth. 2011;58:560–8.
Harris K, Chalhoub M, Maroun R, Elsayegh D. Endotracheal tube exchangers: should we look for safer alternatives? Heart Lung. 2012;41:67–9.
O’Sullivan TJ, Healy GB. Complications of Venturi jet ventilation during microlaryngeal surgery. Arch Otolaryngol. 1985;111:127–31.
Baraka AS. Tension pneumothorax complicating jet ventilation via a cook airway exchange catheter. Anesthesiology 1999;91:557–8.
Eley V, Lloyd B, Scott J, Greenland K. Availability of difficult airway equipment to rural anaesthestists in Queensland, Australia. Rural Remote Health. 2008;8:1020–9.
Porhomayon J, El-Solh AA, Nader ND. National survey to assess the content and availability of difficult airway carts in critical-care units in the United States. J Anesth. 2010;24:811–4.
Oto J, Sun MQ, Kacmarek RM, Jiang Y. Efficacy of ventilation through a customized novel cuffed airway exchange catheter: a tracheal/lung model study. Br J Anaesth. 2014;112:948–9.
Oto J, Su Z, Duggan M, Wang J, King DR, Kacmarek RM, Jiang Y. Efficacy of coaxial ventilation with a novel endotracheal catheter equipped with a functional cuff: a swine model study. Eur J Anaesthesiol. 2016;33:250–6.
Carter EL, Duguid A, Ercole A, Matta B, Burnstein RM, Veenith T. Strategies to prevent ventilation-associated pneumonia: the effect of cuff pressure monitoring techniques and tracheal tube type on aspiration of subglottic secretions: an in-vitro study. Eur J Anaesthesiol. 2014;31:166–71.
Buise M, van Bommel J, van Genderen M, Tilanus H, van Zundert A, Gommers D. Two-lung high-frequency jet ventilation as an alternative ventilation technique during transthoracic esophagectomy. J Cardiothorac Vasc Anesth. 2009;23:509–12.
Elkassabany N, Garcia F, Tschabrunn C, Raiten J, Gao W, Chaichana K, Dixit S, Speck RM, Zado E, Marchlinski F, Mandel J. Anesthetic management of patients undergoing pulmonary vein isolation for treatment of atrial fibrillation using high-frequency jet ventilation. J Cardiothorac Vasc Anesth. 2012;26:433–8.
Raiten J, Elkassabany N, Gao W, Mandel JE. Medical intelligence article: novel uses of high frequency ventilation outside the operating room. Anesth Analg. 2011;112:1110–3.
Funding
Departmental funding. Dr. Liu received the Postdoctoral Science Foundation of Heilongjiang Province, China Grant (No: LBH-Z12206).
Author information
Authors and Affiliations
Contributions
JW designed the study, collected and analyzed the data, and wrote the manuscript. ZS and JO analyzed the data and wrote the manuscript. KRM, YJ and SL designed the study, analyzed the data and wrote the manuscript. All the authors have read and approved the final version of the manuscript to be published.
Corresponding author
Ethics declarations
Conflict of interest
Robert Kacmarek is a consultant for Medtronic, and Orange Medical and has received research grants from Medtronic and Venner Medical.
Rights and permissions
About this article
Cite this article
Wang, J., Su, Z., Oto, J. et al. Endotracheal catheter equipped with functional cuff produces clinically relevant positive end expiratory pressure: a bench study. J Clin Monit Comput 33, 419–429 (2019). https://doi.org/10.1007/s10877-018-0189-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-018-0189-1