Abstract
Transient stop-flow arm arterial–venous equilibrium pressure (Pmsf-arm) is a validated technique for measuring the mean systemic filling pressure (Pmsf). Pmsf is a functional measure of the effective intravascular volume status. This study aims to assess the precision of the Pmsf-arm measurement. Pmsf-arm was measured by inflating a pneumatic tourniquet around the upper arm 50 mmHg above systolic pressure for 60 s, four times consecutively, with an interval of 5 min. Arterial (Pa) and venous pressure (Pv) were recorded every 10 s. Pa–Pv difference was calculated to determine the stop-flow time. The coefficient error (CE) was determined and used to derive the least significant change (LSC) in Pmsf-arm that this technique could reliably detect. The rANOVA test was used to compare repeated measurements of the four determinations of Pmsf-arm. 80 measurements of Pmsf-arm were studied in 20 patients. Pa and Pv equalised after 60 s of inflation (Pa–Pv difference 0 ± 0.01 mmHg). There were no significant differences of Pmsf-arm values among determinations. For a single measurement, the CE was 5 % (±2 %) and the LSC was 14 % (±5 %). Averaging two, three and four measurements the CE improves to 4 % (±1 %), 3 % (±1 %) and 3 % (±1 %) respectively, and the LSC was reduced to 10 % (±4 %), 8 % (±3 %) and 7 % (±3 %) respectively. One measurement of Pmsf-arm can reliably detect changes on Pmsf-arm of 14 %. The precision of Pmsf-arm technique improves when averaging two or three measurements.
Similar content being viewed by others
1 Introduction
In critically ill patients, the accurate assessment of intravascular volume is difficult. Hemodynamic variables such as arterial pressure, central venous pressure (CVP) or cardiac output (CO) can provide indirect information but can be easily misinterpreted as these variables are constantly affected by cardiac function. Dynamic variables obtained from heart–lung interactions are able to identify fluid responsiveness, but this condition can coexists with hypovolaemia, euvolaemia or fluid overload. That said, the assessment of intravascular volume status and its change in response to therapy is essential for the appropriate hemodynamic management of these patients.
The mean systemic filling pressure is (Pmsf) is a quantitative measure of intravascular volume. It is the pressure in the cardiovascular system when there is no blood motion [1] and depends on the “stressed” volume, which is the part of blood volume that generates pressure, and the mean compliance of the cardiovascular system. This physiological variable together with the right atrial pressure and the resistance to venous return determines CO [2].
The mean systemic filling pressure (Pmsf) can be measured in ventilator dependent patients using inspiratory-hold maneuvers defining CVP-CO data pairs that when extrapolated to zero CO (no flow) approximates the Pmsf [3, 4]. It can be also estimated using a mathematical algorithm based on the mean arterial pressure, CVP and CO [5]. Anderson [6] proposed a non-invasive technique to measure Pmsf by a rapid occlusion of the circulation in the arm (Pmsf-arm). Once the arterial (Pa) and venous pressures (Pv) in the arm equilibrate, the pressure measured would be Pmsf. Maas et al. [7] demonstrated that this method was interchangeable with the Pmsf-hold, and useful to track changes in volume status. However, in order to use this method in clinical and research practice, it is important to know the precision of the technique.
Precision can be defined as the degree to which repeated measurements under steady conditions show the same results [8]. Since repeated measurements of Pmsf-arm may bring different results, it is important to know if the changes are related to technical limitations or to real changes of Pmsf, and also whether by repeating the measurement a number of times under steady conditions, we can improve the precision of the measurement. The ability of a technique to detect changes in physiological variables can be measured by the least significant change (LSC). It allows the user of the technique to identify if a real change has actually happened with 95 % of probability, or whether the changes observed were just the result of a random variation. We hypothesize that repeated measurements of Pmsf-arm in hemodynamically stable patients should show similar results, and that the changes observed are related to the lack of precision of this technique. Neither the LSC nor the number of measurements required to generate both an acceptable and a reliable level of precision have been studied for the Pmsf-arm. Therefore, the objective of this study is to elucidate the LSC of the Pmsf-arm measurement, and the number of measurements needed to get a reliable result.
2 Methods
This study was approved by the Camden & Islington ethic committee (13/LO/1307, December 2013) and by St. George’s University of London. Written informed consent was obtained from all patients.
2.1 Participants
Patients admitted to cardiothoracic intensive care unit (ICU) following cardiac surgery were prospectively enrolled. Patients with occlusive peripheral vascular disease, post-operative valve regurgitation, tachyarrhythmia, presence of an intra-aortic balloon pump, pregnancy, body weight below 50 kg and patients requiring fluid resuscitation or changes in vasoactive therapy were excluded from the study. All patients were studied during the initial period in the ICU, once they achieved hemodynamic and respiratory stability, defined by changes no >10 % in heart rate, blood pressure and arterial saturation of oxygen during 10 min.
2.2 Measurements
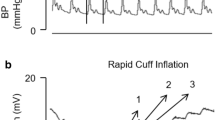
Patients were monitored with invasive radial Pa and all subjects also had a peripheral venous cannula in the forearm. Pressure transducers were connected to arterial and venous cannula and referenced to the level of the forearm. Patients were lying fully supine in bed during the study period. Pa and Pv were then measured in the same hand and upper extremity blood stop-flow was created using a pneumatic tourniquet (AT4 pneumatic tourniquet, AneticAid, Leeds, UK) to pressures 50 mmHg above systolic pressure and held occlusion for 60 s. Measurements were performed four times consecutively, every 5 min in each patient. Pa and Pv values were recorded every 10 s. The absolute difference between Pa and Pv was calculated in order to determine the adequate stop-flow time.
2.3 Statistical analysis
Data are described as means (standard deviation SD) when normally distributed, and medians (interquartile range IQR) otherwise. Normality was assessed using Shapiro–Wilk test. We used similar methodology for the analysis of the precision to that described by Cecconi et al. [9]. Every participant generated a set of four measurements. The coefficient of variation (CV) for each set of Pmsf-arm measurements was estimated as described by Bland and Altman [10]. For a series of replicates CV is calculated as
As the standard deviation (SD) Pmsf-arm decreases as the magnitude of Pmsf-arm increases, a log transformation was used prior to calculation of CV in order to be able to observe the variability unrelated to the magnitude of Pmsf-arm [10]. Log transformation was applied to each set of measurements. The SD of the logarithmic transformations was calculated and then antilog-transformed to calculate the CV for each set of measurements. The mean of the CVs obtained from each study was then calculated. The standard error (SE) is calculated as follows:
SE is used instead of SD when studying the variation of repeated measurements, then the coefficient error (CE) is obtained, where CE = SE/meanPmsf-arm. Therefore, the CE for n number of replicates was calculated. Precision defined as 95 % confidence is 2.7 SD. In terms of percentage, precision is 2.77 CV for single measurements and 2.77 CE for averaged measurements. LSC was calculated using the following equation [11]:
It was considered a priori that a LSC of 15 % was the minimum that could be accepted for clinical practice. Finally, the data on the variability and precision within participant are summarized and reported.
In order to detect if the technique by itself generates changes in the vascular properties that may affect Pa, Pv or Pmsf-arm value, repeated-measures ANOVA test (rANOVA) was used to compare the mean values across the four determinations. Sphericity was not assumed and degrees of freedom were corrected using Greenhouse-Geisser estimates of sphericity (ε). Statistical analysis was performed using SPSS software (IBM SPSS Statistics 21) and GraphPad Prism 6.0. For all comparisons, a p value of <0.05 was considered significant.
3 Results
23 patients were consented. 2 patients were excluded due to a decrease of blood pressure over the study period of more than 10 %, and one because the arterial line was inserted by the anaesthesiologist in the brachial artery. 80 measurements of Pmsf-arm were completed in 20 patients. The patient’s characteristics are presented in Table 1. The cuff achieved the target pressure in 1.48 ± 0.3 s.
3.1 Stop flow time
Data of Pa and Pv after cuff inflation for each measurement are presented in Table 2. The mean values of the differences between Pa and Pv at each 10, 20, 30, 40, 50 and 60 s are 32.4 ± 15.3, 18.1 ± 9.9, 8 ± 5.1, 3.5 ± 3.5, 1.2 ± 2.9 and 0.4 ± 2.4 mmHg respectively (Fig. 1) for one measurement. Similar values are observed over the four measurements (Table 2). Since the smallest Pa–Pv difference was observed after 60 s of cuff inflation, we chose the 60-s value of the Pa as Pmsf-arm for this study.
3.2 Precision of Pmsf-arm measurement
Baseline measurements of Pa and Pv were compared an no significant differences were observe across the four measurements for Pa (ε = 0.17, F (1.77, 33.56) = 1.07, p = 0.35) and for Pv (ε = 0.78, F(2.34, 44.52) = 1.84, p = 0.16; Fig. 2).
The mean value of Pmsf-arm was 22.4 ± 7.7 mmHg. The mean CE (or CV) for a single measurement was 5 % (±2 %). If the mean of two, three or four measurements were used, the CE was reduced to 4 % (±1 %), 3 % (±1 %) and 3 % (±1 %) respectively (Fig. 3). The LSC was 14 % (±5 %) for a single measurement. If the average of two, three or four measurements were used, the LSC was reduced to 10 % (±4 %), 8 % (±3 %) and 7 % (±3 %) respectively (Fig. 3).
Bland–Altman analysis was performed for the first two measurements. We observed a bias of −0.1 ± 1.68 mmHg, 95 % limits of agreement from −3.4 to 3.2 mmHg (Fig. 4). When mean Pmsf-arm values were compared, there were no significant differences across the four measurements (ε = 0.84, F (2.51, 47.73) = 1.73, p = 0.18).
4 Discussion
This study demonstrates that one measurement of the arterial–venous equilibrium pressure has sufficient precision to detect significant changes in Pmsf-arm. In addition, our data showed that arterial–venous equilibrium pressure is achieved at 60 s after cuff inflation and repetition of this technique every 5 min does not alter the baseline values.
The Pmsf-arm technique has been validated in a similar population with a good level of agreement between this technique and inspiratory-hold method for calculation of Pmsf [7]. Validation studies on agreement between two techniques are important to establish the accuracy of the measurement technique but do not provide useful information about the precision of any particular method. Maas et al. [7] reported the repeatability of two Pmsf-arm determinations using Bland–Altman analysis in eleven patients, at two different times: base line and after fluid expansion. They reported negligible bias (0.03 ± 1.02 mmHg) and a CV of 5 %, which is the same we observed. The slightly greater bias between two measurements could be due to the differences in the technique or to a higher number of participants in the analysis. Nevertheless, the bias is still negligible for measurements separated by 5 min. Given the high precision of this technique, Pmsf-arm can be used to estimate Pmsf in ventilated and non-ventilated patients at bedside, which is an advantage in compared with the inspiratory-hold method.
4.1 Arm stop-flow time
Pa and Pv equilibrated at 60 s. Maas et al [7] reported a plateau in both Pa and Pv after 20–30 s. They reported a cuff inflation time shorter than observed with a pneumatic tourniquet, and that could possibly explain this delay in equalization. Apart from the different devices used, the measurements are performed in similar way. Anderson [6] suggested that incomplete interruption of blood flow may occur when the equalization does not happen at 30 s and pressures continue to rise. But in our data, the Pv achieves a plateau level in about 30 s while Pa was still falling until 50–60 s when it equalizes with Pv. In addition, we did not observe a further decrement in Pa nor in Pv after 30 s as suggested by Maas et al., although in four cases, we observed a slightly lower Pa than Pv after 40 s over the four determinations.
4.2 Pa and Pmsf-arm
Maas et al. [7] suggested that ischemic-induced vasodilation might take place after 35–40 s. Anderson et al. [12] studied flow-mediated changes and reactive hyperaemia in 29 humans by inflating a cuff in the forearm during 10 min. In order to exclude systemic reflexes or humoral factors (as ischemic mediators) the brachial artery diameter and flow of the contralateral arm was measured in seven subjects while cuff inflation (10 min) and reactive hyperaemia. Flow or arterial diameter did not change in the contralateral arm. Moreover, Stadler et al. [13] observed the time-course of brachial artery diameter changes during 5 min of cuff-induced ischemia in smokers and non-smokers and observed a minimal increase in arterial diameter in non-smokers and a reduction in arterial diameter (vasoconstriction) in smokers. In this study we did not observe decrease Pv after 30 or 40 s whereas Pa was still decreasing due to the cuff inflation. A certain degree of ischemia-induced vasodilation is possible during 1 min, but it does not seem to prevent equalization of Pa and Pv. In addition, our participants were on (or recently received) propofol and morphine or alfentanyl, which may dampen the sympathetic activity [14–16], thus the involvement of systemic reflexes over the study period is probably low.
4.3 LSC and Pmsf
The precision of this technique is important to study the changes in volume status. A measurement of Pmsf with a CV of 5 % implies that real values will be in a range of 10 % with 95 % confidence. If we took our sample as an example, a Pmsf-arm of 22 mmHg could represent any value from 19.8 to 24.2 mmHg. When we observe the effect of therapy, we can only trust changes above the LSC. Changes below the LSC may not represent a real change. Following the example, a LSC of 14 % in this study means that we need to observe a change of at least ±3.1 mmHg for one determination of Pmsf. With two determinations, LSC decreases to 10 %, and the changes observed will need to be at least ±2.2 mmHg. As we can only observe changes in units of 1 mmHg, we may need 4 mmHg of change in Pmsf-arm measured with one determination, or 3 mmHg with two, to claim a real change with a 95 % of confidence. Three determinations will be able to reduce this LSC interval to ±1.8 mmHg, and four determinations to ±1.5 mmHg. However, as we will not detect those differences with our pressure transducers, we can approximate that three determinations will be enough to reliably detect a 2 mmHg change in Pmsf-arm for a Pmsf-arm of 22 mmHg.
4.4 Methodological issues and other study limitations
During a vascular occlusion, large arteries tone is affected by changes in blood flow (flow-mediated dilation), which is independent of α or β adrenergic receptors and cholinergic mechanisms [12, 17]. This can alter the repeatability of the measurements. Hintze et al. [17] studied the flow-mediated dilatation of coronary arteries in dogs, and observed that brief periods of coronary artery occlusion (15 s) generate reactive vasodilation of large arteries after the occlusion release, it reaches a peak after 1 min and returns to baseline level in about 5 min after the occlusion release. Sinoway [18] reported that occlusion of the brachial artery (in humans) for 1 min generates an increase in blood flow, arterial cross sectional area and diameter after release of the occlusion. The time pattern for an occlusion of 3 min was: artery diameter did not reach the peak value until 15 s after occlusion release, and the reduction in diameter was described by a first order rate constant 0.019 cm s−1 (baseline diameter 0.29 cm), and a half time for diameter reduction of 35.8 s. Maas et al. [19] observed Pmsf-arm during a 50 mL stepwise fluid administration protocol, measuring Pmsf-arm ten times every 2 min in 15 patients. Although the baseline values of each inflation was not reported, the authors stated that the Pmsf-arm–Volume relationship was linear and therefore inflations repeated every 2 min did not affect the vascular tone allowing the observation of volume changes. In the present study, spacing the measurements by 5 min, we did not observe significant changes in the Pa or Pv values at 60 s of vascular occlusion, so we suspect that after 5 min of occlusion release, regional blood flow and arterial and venous tone normalize.
Second, we assume that any changes detected were from the technique itself rather than real changes in Pmsf due to, for instance, changes in the volume status or vascular tone. Unfortunately we cannot control those variables but, to minimize this potential risk, we performed the four measurements in as short a time as possible (20 min). We also studied only patients hemodynamically stable. When blood pressure decreased over the study period at baseline, requiring further fluids or changes in vasoactive medications, the patient was excluded from the analysis. The variability observed may be significantly higher in unstable patients, making the technique less robust unless more measurements are used to get the averaged value. It is possible that changes in vascular tone in the arm are not detected by the usual haemodynamic parameters. We did not observe either any significant difference between Pa and Pv at baseline over the four measurements.
Third, significant differences between aortic pressure and radial pressure have been reported after cardiopulmonary bypass [20, 21], although those differences disappear after about 60 min [20]. This study was performed once hemodynamic stability was achieved, approximately 2–3 h after discontinuation of cardiopulmonary bypass.
Fourth, although the values of Pmsf that we described are consistent with recent studies in similar patients [3, 7, 19, 22] it is possible that we are overestimating Pmsf. First, cuff inflation time of the pneumatic tourniquet is longer than previously reported by Maas et al. [19]. In this time there is a brief cessation of venous return prior to arterial stop-flow equal to 1.5–2.4 heartbeats assuming heart rates between 60 and 100 bpm. We expect this overestimation to be negligible because the amount of inflow would be relatively small compared to the total distal arm blood volume. Second, certain amount of blood is displaced into the vascular tree in the arm when the cuff is inflated. This may be also one of the reasons why equalisation between Pa and Pv takes longer than in Maas et al. studies.
There are some other limitations to this study. We deliberately did not include children or septic patients. The results of this study, therefore, could be different when extrapolated to other patient groups.
Second, our sample size is relatively small. We performed an interim analysis with 10 patients and we observed similar results, therefore we believe that further increment in sample size will not change significantly our results.
Finally, although we explained the clinical implications of our results, more studies are needed in order to assess the effect of treatments on Pmsf-arm. We decided to report the results of this study separately for the sake of simplicity and comprehensibility and to stress the methodological aspects.
5 Conclusion
The precision (calculated as CV) of transient stop-flow arm arterial–venous equilibrium technique to measure Pmsf for one measurement is 5 % and the LSC is 14 %. These values improve when averaging two or three measurements. This technique will enable a high level of precision, which will allow small but significant changes in Pmsf to be detected at bedside and observe the effect of fluids or vasoactive medications on the intravascular fluid status and vascular tone.
References
Bayliss WM, Starling EH. Observations on venous pressures and their relationship to capillary pressures. J Physiol. 1894;16(3–4):159–318.
Guyton AC. Determination of cardiac output by equating venous return curves with cardiac response curves. Physiol Rev. 1955;35(1):123–9.
Maas JJ, Geerts BF, van den Berg PC, Pinsky MR, Jansen JR. Assessment of venous return curve and mean systemic filling pressure in postoperative cardiac surgery patients. Crit Care Med. 2009;37(3):912–8. doi:10.1097/CCM.0b013e3181961481.
Jansen JR, Maas JJ, Pinsky MR. Bedside assessment of mean systemic filling pressure. Curr Opin Crit Care. 2010;16(3):231–6. doi:10.1097/MCC.0b013e3283378185.
Parkin WG, Leaning MS. Therapeutic control of the circulation. J Clin Monit Comput. 2008;22(6):391–400. doi:10.1007/s10877-008-9147-7.
Anderson RM (1993) The gross physiology of the cardiovascular system, 2012th edn. Racquet Press, Tucson
Maas JJ, Pinsky MR, Geerts BF, de Wilde RB, Jansen JR. Estimation of mean systemic filling pressure in postoperative cardiac surgery patients with three methods. Intensive Care Med. 2012;38(9):1452–60. doi:10.1007/s00134-012-2586-0.
(JCGM/WG2) WGJCfGiM (2008) JCGM 200:2008 International vocabulary of metrology—basic and general concepts and associated terms (VIM), 3rd edn. JCGM
Cecconi M, Dawson D, Grounds RM, Rhodes A. Lithium dilution cardiac output measurement in the critically ill patient: determination of precision of the technique. Intensive Care Med. 2009;35(3):498–504. doi:10.1007/s00134-008-1292-4.
Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135–60.
Lodder MC, Lems WF, Ader HJ, Marthinsen AE, van Coeverden SC, Lips P, Netelenbos JC, Dijkmans BA, Roos JC. Reproducibility of bone mineral density measurement in daily practice. Ann Rheum Dis. 2004;63(3):285–9.
Anderson EA, Mark AL. Flow-mediated and reflex changes in large peripheral artery tone in humans. Circulation. 1989;79(1):93–100.
Stadler RW, Ibrahim SF, Lees RS. Measurement of the time course of peripheral vasoactivity: results in cigarette smokers. Atherosclerosis. 1998;138(1):197–205.
Frithiof R, Rundgren M. Activation of central opioid receptors determines the timing of hypotension during acute hemorrhage-induced hypovolemia in conscious sheep. Am J Physiol Regul Integr Comp Physiol. 2006;291(4):R987–96. doi:10.1152/ajpregu.00070.2006.
Sato M, Tanaka M, Umehara S, Nishikawa T. Baroreflex control of heart rate during and after propofol infusion in humans. Br J Anaesth. 2005;94(5):577–81. doi:10.1093/bja/aei092.
Lennander O, Henriksson BA, Martner J, Biber B. Effects of fentanyl, nitrous oxide, or both, on baroreceptor reflex regulation in the cat. Br J Anaesth. 1996;77(3):399–403.
Hintze TH, Vatner SF. Reactive dilation of large coronary arteries in conscious dogs. Circ Res. 1984;54(1):50–7.
Sinoway LI, Hendrickson C, Davidson WR Jr, Prophet S, Zelis R. Characteristics of flow-mediated brachial artery vasodilation in human subjects. Circ Res. 1989;64(1):32–42.
Maas JJ, Pinsky MR, Aarts LP, Jansen JR. Bedside assessment of total systemic vascular compliance, stressed volume, and cardiac function curves in intensive care unit patients. Anesth Analg. 2012;115(4):880–7. doi:10.1213/ANE.0b013e31825fb01d.
Stern DH, Gerson JI, Allen FB, Parker FB. Can we trust the direct radial artery pressure immediately following cardiopulmonary bypass? Anesthesiology. 1985;62(5):557–61.
Rich GF, Lubanski RE Jr, McLoughlin TM. Differences between aortic and radial artery pressure associated with cardiopulmonary bypass. Anesthesiology. 1992;77(1):63–6.
Maas JJ, Pinsky MR, de Wilde RB, de Jonge E, Jansen JR. Cardiac output response to norepinephrine in postoperative cardiac surgery patients: interpretation with venous return and cardiac function curves. Crit Care Med. 2013;41(1):143–50. doi:10.1097/CCM.0b013e318265ea64.
Acknowledgments
Hollmann D. Aya received financial support for educational programs and for attending symposia from Applied Physiology, LiDCO. Andrew Rhodes has received honoraria for advisory board for LiDCO, honoraria for Covidien, Edwards Lifesciences and Cheetah. Maurizio Cecconi has received honoraria for speaking at symposia, financial support for educational programs and honoraria for advisory board from Edwards Lifesciences, LiDCO, Deltex, Applied Physiology, Massimo, Bmeye, Cheetah, Imacor.
Ethical standard
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research ethical committee (NRES Committee London—Camden & Islington, Health Research Authority, National Research Ethics Service (NRES) Reference: 13.LO.1307) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aya, H.D., Rhodes, A., Fletcher, N. et al. Transient stop-flow arm arterial–venous equilibrium pressure measurement: determination of precision of the technique. J Clin Monit Comput 30, 55–61 (2016). https://doi.org/10.1007/s10877-015-9682-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-015-9682-y