Abstract
Introduction
Placing EMG electrode pairs that span several muscles is sometimes used to enhance the efficacy of electromyographic recordings. This technique, often referred to as “jumping,” has not been studied when using Motor Evoked Potentials (TcMEP) for detecting isolated spinal nerve root injury during spine surgery.
Methods
TcMEPs were obtained in seven pigs under general anesthesia. One pair of recording electrodes was placed entirely within the tibialis anterior (TA) muscle; a second pair had one lead in the TA and the other in the gastrocnemius muscle (TA-GAS). The dominant root innervating the TA was determined using evoked EMG. MEP amplitudes recorded by the TA and TA-GAS electrodes were compared before and after suture ligation of this root in 12 separate experiments.
Results
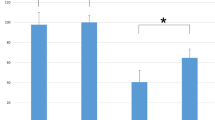
Mean baseline TcMEP amplitude was not significantly different for the TA vs. TA-GAS. After root ligation, mean amplitude dropped from baseline by 72 ± 13% in the TA vs. 50 ± 29% in the TA-GAS (p < 0.01). All amplitudes decreased by >50% in the TA group; half of the TA-GAS group had <50% decrease in amplitude.
Discussion
Mixed-myotomal recording electrodes did not consistently increase baseline TcMEP amplitude. The decrease in amplitude after ligation was both smaller and more variable in the “jumped” TA-GAS electrodes. Thus, this technique may allow someone relying on TcMEP monitoring to miss an otherwise detectable isolated nerve root injury (i.e., have a false-negative result).
Similar content being viewed by others
References
Fan D, Schwartz DM, Vaccaro AR, Hilibrand AS, Albert TJ. Intraoperative neurophysiologic detection of iatrogenic C5 nerve root injury during laminectomy for cervical compression myelopathy. Spine (Phila Pa 1976). 2002;27:2499–502.
Lieberman JA, Lyon R, Feiner J, Hu SS, Berven SH. The efficacy of motor evoked potentials in fixed sagittal imbalance deformity correction surgery. Spine (Phila Pa 1976). 2008;33:E414–24.
Sakaura H, Hosono N, Mukai Y, Ishii T, Yoshikawa H. C5 palsy after decompression surgery for cervical myelopathy: review of the literature. Spine (Phila Pa 1976). 2003;28:2447–51.
Leppanen RE, Abnm D. American society of neurophysiological M. Intraoperative monitoring of segmental spinal nerve root function with free-run and electrically-triggered electromyography and spinal cord function with reflexes and F-responses. A position statement by the American society of neurophysiological monitoring. J Clin Monit Comput. 2005;19:437–61.
Resnick DK, Choudhri TF, Dailey AT, Groff MW, Khoo L, Matz PG, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 15: electrophysiological monitoring and lumbar fusion. J Neurosurg Spine. 2005;2:725–32.
Sutter MA, Eggspuehler A, Grob D, Porchet F, Jeszenszky D, Dvorak J. Multimodal intraoperative monitoring (MIOM) during 409 lumbosacral surgical procedures in 409 patients. Eur Spine J. 2007;16(Suppl 2):S221–8.
Mok JM, Lyon R, Lieberman JA, Cloyd JM, Burch S. Monitoring of nerve root injury using transcranial motor-evoked potentials in a pig model. Spine (Phila Pa 1976). 2008;33:E465–73.
Woodforth IJ, Hicks RG, Crawford MR, Stephen JP, Burke DJ. Variability of motor-evoked potentials recorded during nitrous oxide anesthesia from the tibialis anterior muscle after transcranial electrical stimulation. Anesth Analg. 1996;82:744–9.
Guo L, Jasiukaitis P, Pitts LH, Cheung SW. Optimal placement of recording electrodes for quantifying facial nerve compound muscle action potential. Otol Neurotol. 2008;29:710–3.
Jonas D, Bischoff C, Conrad B. Influence of different types of surface electrodes on amplitude, area and duration of the compound muscle action potential. Clin Neurophysiol. 1999;110:2171–5.
Fuglevand AJ, Winter DA, Patla AE, Stashuk D. Detection of motor unit action potentials with surface electrodes: influence of electrode size and spacing. Biol Cybern. 1992;67:143–53.
Zedka M, Kumar S, Narayan Y. Comparison of surface EMG signals between electrode types, interelectrode distances and electrode orientations in isometric exercise of the erector spinae muscle. Electromyogr Clin Neurophysiol. 1997;37:439–47.
Moller AR. Intraoperative neurophysiologic monitoring. Am J Otol. 1995;16:115–7.
Pechstein U, Cedzich C, Nadstawek J, Schramm J. Transcranial high-frequency repetitive electrical stimulation for recording myogenic motor evoked potentials with the patient under general anesthesia. Neurosurgery. 1996;39:335–43. Discussion 43–4.
Koh TJ, Grabiner MD. Evaluation of methods to minimize cross talk in surface electromyography. J Biomech. 1993;26(Suppl 1):151–7.
Santiago-Perez S, Nevado-Estevez R, Aguirre-Arribas J, Perez-Conde MC. Neurophysiological monitoring of lumbosacral spinal roots during spinal surgery: continuous intraoperative electromyography (EMG). Electromyogr Clin Neurophysiol. 2007;47:361–7.
Dimitrova N. Model of the extracellular potential field of a single striated muscle fibre. Electromyogr Clin Neurophysiol. 1974;14:53–66.
Lynn PA, Bettles ND, Hughes AD, Johnson SW. Influences of electrode geometry on bipolar recordings of the surface electromyogram. Med Biol Eng Comput. 1978;16:651–60.
Romstock J, Strauss C, Fahlbusch R. Continuous electromyography monitoring of motor cranial nerves during cerebellopontine angle surgery. J Neurosurg. 2000;93:586–93.
Katz J. Handbook of clinical audiology. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2001.
Bose B, Wierzbowski LR, Sestokas AK. Neurophysiologic monitoring of spinal nerve root function during instrumented posterior lumbar spine surgery. Spine (Phila Pa 1976). 2002;27:1444–50.
Liguori R, Krarup C, Trojaborg W. Determination of the segmental sensory and motor innervation of the lumbosacral spinal nerves. An electrophysiological study. Brain. 1992;115(Pt 3):915–34.
Young A, Getty J, Jackson A, Kirwan E, Sullivan M, Parry CW. Variations in the pattern of muscle innervation by the L5 and S1 nerve roots. Spine (Phila Pa 1976). 1983;8:616–24.
Author information
Authors and Affiliations
Corresponding author
Additional information
Lyon R, Burch S, Lieberman J. Mixed-muscle electrode placement (“jumping” muscles) may produce false-negative results when using transcranial motor evoked potentials to detect an isolated nerve root injury in a porcine model.
Rights and permissions
About this article
Cite this article
Lyon, R., Burch, S. & Lieberman, J. Mixed-muscle electrode placement (“jumping” muscles) may produce false-negative results when using transcranial motor evoked potentials to detect an isolated nerve root injury in a porcine model. J Clin Monit Comput 23, 403–408 (2009). https://doi.org/10.1007/s10877-009-9205-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-009-9205-9