Abstract
A polyhydrido copper nanocluster, [Cu14H10(PPh3)8(SR)3]+ (HSR = 2,4-dimethylbenzenethiol), adopting a distorted fcc structure, is reported. One cube-vertex copper atom, coordinated by the three thiolate ligands and a PPh3, protrudes outwards from the fcc metal framework. The twisting of the three thiolate ligands about the threefold axis lowers the symmetry from Oh (Cu14) to C3, forming racemic pairs of intrinsic chiral clusters in crystalline solid-state.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metal nanoclusters (NCs) are developing rapidly as a new class of nanomaterials with atomically precise structures and potential applications in various fields, providing a bridge between atoms and nanoparticles [1,2,3,4]. In recent years, the coinage-metal NCs with different sizes have been reported [5,6,7,8,9]. Due to the relatively instability of copper (due to its lower half-cell potential), synthesis and isolation of copper nanoclusters are rather challenging. Morphologically, the regiospecific arrangements, such as convex vertices and variously oriented facets, in anisotropic nanoclusters are the main functional sites [10]. Investigation of the structure and bonding characteristics of atom-precise metal nanoclusters is a prerequisite in the design and synthesis of functional nanomaterials such as catalysts [11,12,13].
Despite their fascinating complex architectures, [14,15,16,17,18] coinage clusters with regular standard unit skeleton, such as the 14-atom fcc, 9-atom bcc and 17-atom hcp unit, are rarely reported. Only one gold cluster [Au9(PPh3)8]+ exhibiting perfect bcc unit was reported [19]. While for silver clusters, a series of Ag14 exhibiting discrete fcc unit were synthesized by monodentate-thiolate ligand [20, 21], in which the eight metal vertices were occupied by [(SR)3Ag] (SR: thiolate) motifs. In contrast, with bidentate-thiolate ligand, a Ag14 showing the typical fcc compact fashion with argentophilic Ag···Ag interactions was isolated [22]. Further engineering the growth of the clusters by ligand-control, a family of fcc silver nanoclusters, from Nichol’s to Rubik’s cube and beyond, were successfully prepared [23]. To better understand the underlying mechanism of nanoparticles growth, it is important to synthesize analogues or congeners of these clusters with simple ligand units. As part of the fcc core, the octahedral [CuHPPh3]6 has been known for almost half century [24, 25]. In 2020, Zang’s group reported an alkynyl copper cluster with quasi-fcc 14-Cu skeleton [26], in which a distorted octahedron Cu66+ unit was encapsulated in Cu88+ cage. Interestingly, there were no metallic interactions between Cu66+ and Cu88+. The same group also reported a dithiolate coordinated Cu14 cluster with regular fcc skeleton27. For larger Cu clusters, a novel Cu36H10 cluster with half Nichol’s cube was reported by Bakr’s group [28].
More recently, Shen’s group reported another 14-Cu cluster, formulated a [Cu14H10(PPh3)7(SR)3]+ (1) [29] where SR = StBu. This work prompted us to report our independent work on the synthesis and crystal structure of a related but distinct polyhydrido copper cluster, formulated as [Cu14H10(PPh3)8(SR)3]+ (2), where SR = 2,4-dimethylbenzenethiolate. The metal framework of both clusters adopts similar distorted fcc structure of C3 symmetry, but with different numbers of phosphine ligands (seven in 1 and eight in 2). Both clusters form racemic pairs of intrinsic chiral clusters in solid-state.
Results and Discussions
The title cluster was synthesized by reducing a yellow mixture of Cu(OAc)2•2H2O, 2,4-dimethylbenzenethiol (RSH), PPh3 and Ph4PBPh4 in CH2Cl2 with aqueous NaBH4 (see detailed synthetic procedure in the SI). The as-synthesized bright yellow product was then purified by washing with methanol and water. And cuboid yellow crystals suitable for single-crystal X-ray diffraction (SCXRD) were grown from dichloromethane solution after about 7 days.
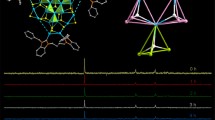
The title cluster crystallized in a cubic unit cell of space group P\(a\stackrel{-}{3}\) (CCDC 2,265,075) with eight formula units of [Cu14H10(PPh3)8(SPhMe2)3]+. The cationic cluster is intrinsically chiral and exists as racemic pairs in the unit cell. As shown in Figs. 1 and 2, the cationic cluster consists of a fcc Cu14 core passivated by eight PPh3 and three thiolate ligands. Figure 3 is a layer-by-layer representation of the Cu14H10S3 core of 2, as viewed along the C3 axis. Here Cua(1), Cub(3), Cuc(3), Cud(1) constitute the cube and Cue(3) and Cuf(3) are the face centers. The eight PPh3 are coordinated to the eight cubic Cu vertices (one each). Interestingly, one unique Cu atom, denoted as Cua, is discretely protruded outwards. As a result, the Oh symmetry is lowered to (idealized) C3 (viz., Figs. 2 and 3). Note that each of the three µ3-S atoms not only are coordinated to the protruded vertex Cua, but also to one adjacent vertex, Cub, and one face center, Cue.
The Cu-Cu bonds distances are listed in Table S1. They range from 2.42 Å to 2.97 Å (av. 2.61 Å), somewhat longer than that of 2.56 Å in copper metal, indicating weak bonding interactions.26 A twist of the three thiolates by 26 degrees about the C3, in either clockwise or counter-clockwise directions, endows the cluster with intrinsic chirality (see also Fig. S1, S2, and S3), giving rise to racemic pairs of R and S enantiomers (Fig. S3a, b) in unit cell. The average Cu-P and Cu-S distances are 2.23, 2.34 Å, respectively, comparable to the corresponding values in 1. A detailed comparison of the Cu-Cu distances in clusters 1 and 2 (cf. Table S1) revealed that the former (with 7 PPh3) is closer to a normal fcc structure, while the latter (with 8 PPh3) has one of the cube vertices (Cua in Fig. 3) extruded. This interplay of the steric effects and the bonding interactions in the observed structure, which represents a compromise in nature, is rather intriguing.
It is interesting to compare the following four Cu clusters structures: [CuHPPh3]6 (with octahedral core only), Zang’s quasi-fcc 14-Cu cluster, 2 (this work), and Zang’s fcc 14-Cu cluster. It shows progressive interactions of the outer Cu8 cube with the inner octahedral Cu6 core, as shown as in Fig. S4. When steric 2-diphenyl-2-hydroxylmethylpyrrolidine-1-propyne was used in the synthesis, the 14-Cu skeleton were stretched to form discrete, noninteracting Cu6 core and Cu8 shell [26]. With Ph3P and monodentate thiol as in 2 (this work), the core-shell interaction enhances (except the protruded Cu vertex). Finally, when suitable bidentate thiol was utilized in the synthesis, a normal fcc 14-Cu cell was obtained with acetonitrile coordinated to the cube vertices [27]. This phenomenon of ligand-triggered progressive, stepwise enhancement of core-shell interaction may be useful in controlling metal cluster bonding and hence in tuning their properties.
Further comparison of 1 and 2, as depicted in Fig. S5, showed that 2 has weak intracluster C − H‧‧‧π interactions involving PPh3 and thiolates around the protruded vertex, while 1 exhibits severe steric hindrance with short C-H‧‧‧H-C distances. This may be the reason why 1 has one less PPh3 than 2 (i.e., the severe steric hindrance leaves no space for the 8th PPh3 to coordinate to the protruded Cu vertex).
ESI-MS, in the positive ionization mode, was carried out to determine the number of hydrides. The distinct peak at m/z 3408.95 and the isotope distribution allowed the formulation of the cluster as [Cu14H10(PPh3)8(SPhMe2)3]+ monocation with ten hydrides, as shown in Fig. 4. Following the strategy for investigation of the hydride locations in metal clusters [30,31,32,33], we also synthesized the deuterated analogue of the cluster by using NaBD4 as the reducing agent. ESI-MS result of the deuteride cluster shows a 10-mass-unit shift of the molecular peak at m/z 3419.09 attributed to [Cu14D10(PPh3)8(SPhMe2)3]+, denoted as Cu14D10 ( 2D, see Fig. S6).
To determine the locations of 10 hydrides, analysis of bonds evaluation, 2 H-NMR spectroscopy and single crystal XRD at low temperature were carried out [30,31,32,33]. The 2 H-NMR spectrum (Fig. 5) of the cluster was recorded by an 850 MHz spectrometer, which clearly showed four distinct peaks with intensity ratios of 1:3:3:3. This strongly suggests the presence of ten D atoms in the cluster with four different environments, at least on the NMR time scale.
Taking the consideration of shorter Cu-Cu bonds evaluation (as shown in Fig. S7), symmetry and validation against the peaks obtained in the difference electron density map of the crystallographic data collected at low temperature (this work and others [30, 31]), the locations of the 10 hydrides were determined as shown in Fig. 3: the centered one (as Ho) adopts a µ6-coordination connected to the six face-center copper atoms (see Fig. S8a); three µ3-H (as Ha) facially coordinates one face-center and two cube vertices (Cu atoms) opposite to the thiolated cubic vertex, as shown in Fig. S8b; three µ4-H (as Hb) coordinated to two face-centers and two cube vertices (Cu atoms) with thiolates, as shown in Fig. S8c); and three µ4-H (as Hc) coordinated to two face-centers and one cube vertex Cu with thiolates (see Fig. S8d). In view of the fact that each fcc unit cell contains one octahedral hole and eight tetrahedral holes, the hydride locations may also be described as follows: Ho(1) is in the octahedral hole; Hb(3) and Hc(3) reside in six “butterfly” (µ4’) holes (which correspond to six of the eight tetrahedral holes); and Ha(3) are face caps. These assignments are fully consistent with the three empirical rules reported recently [30,31,32,33viz., the 2 H-NMR peaks at 11.2, 5.2, 3.6, and 2.8 ppm can be assigned to µ6-Ho, µ4’-Hb, µ4’-Hc, and µ3-Ha, respectively. Here the decreasing downfield shifts correlate well with the decreasing degree of coordination. The Cu-H bonds distances are listed in Table S2.
In general, copper hydrides are active for hydrogenation catalysis [34,35,36,37], we carried out hydrogenation catalytic reactions with different substances, unfortunately, cluster 2 irreversibly released Hs and transformed into amorphous nanoparticles. When heated to 50 ℃, H2 was detected by gas chromatography. The ultraviolet-visible (UV − vis) absorption spectrum of 2 displays much weaker absorption than cluster 1, as shown in Figure S9.
Conclusion
In summary, we have synthesized and fully characterized a [Cu14H10(PPh3)8(SPhMe2)3]+ (2) cluster, which shows a distorted fcc metal unit with one Cu vertex protruded outwards. The protruded vertex is caused by the thiolate coordination, thereby lowering the symmetry of the core from Oh to C3, thereby endowing the cluster with intrinsic chirality, and leading to have one extra PPh3 than cluster [Cu14H10(PPh3)7(SR)3]+ (1) [29]. The cluster is further stabilized by ten hydrides whose locations were determined by SCXRD and substantiated by NMR studies. When heated, the cluster irreversibly releases hydrogen. More work is in progress to carry out the chiral separation and expand the catalytic activity of the cluster under controlled conditions, as well as to explore the growth and/or transformation of these copper hydride nanoclusters in building up the nanomaterials base for future hydrogen technology.
Data Availability
The datasets used or analysed during the current study are available from the corresponding author on reasonable request.
References
I. Chakraborty and T. Pradeep, Chem. Rev., 2017, 117, 8208–8271.
R. C. Jin, C. J. Zeng, M. Zhou and Y. X. Chen, Chem. Rev., 2016, 116, 10346–10413.
R. S. Dhayal, W. E. van Zyl and C. W. Liu, Accounts Chem. Res., 2016, 49, 86–95.
H. Shen, G. L. Tian, Z. Xu, L. Z. Wang, Q. Y. Wu, Y. H. Zhang, B. K. Teo and N. F. Zheng, Coord. Chem. Rev., 2022, 458.
X. H. Ma, J. T. Jia, P. Luo, Z. Y. Wang, S. Q. Zang and T. C. W. Mak, Nano Res., 2022, 15, 5569–5574.
C. P. Joshi, M. S. Bootharaju, M. J. Alhilaly and O. M. Bakr, J. Am. Chem. Soc., 2015, 137, 11578–11581.
M. W. Heaven, A. Dass, P. S. White, K. M. Holt and R. W. Murray, J. Am. Chem. Soc., 2008, 130, 3754–3755.
H. Yang, Y. Wang, H. Huang, L. Gell, L. Lehtovaara, S. Malola, H. Häkkinen and N. Zheng, Nat. Commun., 2013, 4, 2422.
A. Desireddy, B. E. Conn, J. S. Guo, B. Yoon, R. N. Barnett, B. M. Monahan, K. Kirschbaum, W. P. Griffith, R. L. Whetten, U. Landman and T. P. Bigioni, Nature, 2013, 501, 399–402.
B.-L. Han, Z. Liu, L. Feng, Z. Wang, R. K. Gupta, C. M. Aikens, C.-H. Tung and D. Sun, J. Am. Chem. Soc., 2020, 142, 5834–5841.
B. K. Teo, H. Yang, J. Yan and N. Zheng, Inorg. Chem., 2017, 56, 11470–11479.
H. F. Qian, C. Liu and R. C. Jin, Sci. China Chem., 2012, 55, 2359–2365.
Y. M. Su, B. Q. Ji, Z. Wang, S. S. Zhang, L. Feng, Z. Y. Gao, Y. W. Li, C. H. Tung, D. Sun and L. S. Zheng, Sci. China Chem., 2021, 64, 1482–1486.
J. Weßing, C. Ganesamoorthy, S. Kahlal, R. Marchal, C. Gemel, O. Cador, A. C. H. Da Silva, J. L. F. Da Silva, J.-Y. Saillard and R. A. Fischer, Angew. Chem. Int. Ed., 2018, 57, 14630–14634.
M. Qu, F. Q. Zhang, D. H. Wang, H. Li, J. J. Hou and X. M. Zhang, Angew. Chem. Int. Ed., 2020, 59, 6507–6512.
H. Yang, Y. Wang, X. Chen, X. Zhao, L. Gu, H. Huang, J. Yan, C. Xu, G. Li, J. Wu, A. J. Edwards, B. Dittrich, Z. Tang, D. Wang, L. Lehtovaara, H. Hakkinen and N. Zheng, Nat. Commun., 2016, 7, 12809.
N. Yan, N. Xia, L. W. Liao, M. Zhu, F. M. Jin, R. C. Jin and Z. K. Wu, Sci. Adv., 2018, 4, eaat7259.
Y. Li, M. Zhou, Y. Song, T. Higaki, H. Wang and R. Jin, Nature, 2021, 594, 380–384.
H. Shen, E. Selenius, P. P. Ruan, X. H. Li, P. Yuan, O. Lopez-Estrada, S. Malola, S. C. Lin, B. K. Teo, H. Hakkinen and N. F. Zheng, Chem. Eur. J., 2020, 26, 8465–8470.
G. C. Deng, S. Malola, P. Yuan, X. H. Liu, B. K. Teo, H. Hakkinen and N. F. Zheng, Angew. Chem. Int. Ed., 2021, 60, 12897–12903.
H. Y. Yang, J. Lei, B. H. Wu, Y. Wang, M. Zhou, A. D. Xia, L. S. Zheng and N. F. Zheng, Chem. Commun., 2013, 49, 300–302.
Z. Y. Wang, M. Q. Wang, Y. L. Li, P. Luo, T. T. Jia, R. W. Huang, S. Q. Zang and T. C. W. Mak, J. Am. Chem. Soc., 2018, 140, 1069–1076.
H. Y. Yang, J. Z. Yan, Y. Wang, H. F. Su, L. Gell, X. J. Zhao, C. F. Xu, B. K. Teo, H. Hakkinen and N. F. Zheng, J. Am. Chem. Soc., 2017, 139, 31–34.
S.A. Bezman, M.R. Churchill, J.A. Osborn, J. Wormald, J. Am. Chem. Soc., 1971, 93, 2063–2065.
R.C. Stevens, M.R. McLean, R. Bau, T.F. Koetzle, J. Am. Chem. Soc., 1989, 111, 3472–3473;
M.M. Zhang, X.Y. Dong, Z.Y. Wang, H.Y. Li, S.J. Li, X. Zhao, S.Q. Zang, Angew. Chem. Int. Ed., 2020, 59, 10052–10058.
Y.L. Li, J. Wang, P. Luo, X.H. Ma, X.Y. Dong, Z.Y. Wang, C.X. Du, S.Q. Zang, T.C.W. Mak, Adv. Sci., 2019, 6, 1900833–1900839.
C. Dong, R.-W. Huang, C. Chen, J. Chen, S. Nematulloev, X. Guo, A. Ghosh, B. Alamer, M. N. Hedhili, T. T. Isimjan, Y. Han, O. F. Mohammed and O. M. Bakr, J. Am. Chem. Soc., 2021, 143, 11026–11035.
L. Wang, X. Yan, G. Tian, Z. Xie, S. Shi, Y. Zhang, S. Li, X. Sun, J. Sun, J. He and H. Shen, Dalton Trans, 2023, 52, 3371–3377.
X. Yuan, C. Sun, X. Li, S. Malola, B. K. Teo, H. Häkkinen, L.-S. Zheng and N. Zheng, J. Am. Chem. Soc., 2019, 141, 11905–11911.
C. F. Sun, N. Mammen, S. Kaappa, P. Yuan, G. C. Deng, C. W. Zhao, J. Z. Yan, S. Malola, K. Honkala, H. Hakkinen, B. K. Teo and N. F. Zheng, Acs Nano, 2019, 13, 5975–5986.
X. H. Liu, H. Shen, Y. Gao, G. C. Deng, W. H. Deng, Y. Z. Han, B. K. Teo and N. F. Zheng, Chem. Commun., 2022,58, 7670–7673.
C. F. Sun, B. K. Teo, C. L. Deng, J. Q. Lin, G. G. Luo, C. H. Tung and D. Sun, Coord. Chem. Rev., 2021, 427,<vertical-align:sub;> </vertical-align:sub;>213576.
B. H. Lipshutz, Copper-Catalyzed Asymmetric Synthesis. Secondary CuH in asymmetric reductions, Wiley-VCH Verlag GmbH & Co. KGaA, 2014. https://doi.org/10.1002/9783527664573.ch7, pp. 179–202.
C. Deutsch, N. Krause and B. H. Lipshutz, Chem. Rev., 2008, 108, 2916–2927.
Q. Tang, Y. J. Lee, D. Y. Li, W. Choi, C. W. Liu, D. Lee and D. E. Jiang, J. Am. Chem. Soc., 2017, 139, 9728–9736.
C. Y. Liu, S. F. Yuan, S. Wang, Z. J. Guan, D. E. Jiang and Q. M. Wang, Nat. Commun., 2022, 13, 2082.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 21801212), the Scientific Research Funds of Huaqiao University and the Fundamental Research Funds for the Central Universities (Grant No. ZQN-906).
Author information
Authors and Affiliations
Contributions
Cunfa Sun: Conceptualization, Validation, Supervision, Funding Acquisition and Resources; Shuhuan Zeng: Experiments and Data curation; Xin Ge: Investigation and Comparison; Hongwen Deng: Methodology; Shuwei Hao: Visualization; Zhiye Zhang: Analysis and formaiton; Boon K Teo: Writing–Review.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Ethical Approval
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Material
Electronic Supplementary Information (ESI) available: Details of the atomic structure of the cluster 2, their analysis, and simulations in the crystal environment. The data canbe obtained free of charge via.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zeng, S., Ge, X., Deng, H. et al. Synthesis and Structure of Polyhydrido Copper Nanocluster [Cu14H10(PPh3)8(SPhMe2)3]+: Symmetry-Breaking by Thiolate Ligands to form Racemic Pairs of Chiral Clusters in Solid-State. J Clust Sci 35, 109–113 (2024). https://doi.org/10.1007/s10876-023-02469-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-023-02469-w