Abstract
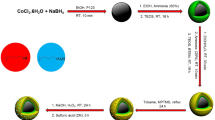
We have developed a biological macromolecule stabilized method for the preparation of cobalt sulfide nanoparticles using carboxymethyl cellouse (CMC) as a stabilizing agent. We investigated the formation of cobalt sulfide nanoparticles as a function of different amounts of CMC. At 0.05% w/v amount of CMC, the high percentage of cube shaped cobalt sulfide nanoparticles is obtained. The samples obtained from 0.01% w/v and 0.1% w/v amounts of CMC shows random shaped cobalt sulfide nanoparticles and aggregated cobalt sulfide nanostructures, respectively. The obtained cobalt sulfide nanoparticles are stabilized through the interactions of hydroxyl (–OH) and carboxylate (–COO–) functional groups in CMC. The CMC encapsulated cobalt sulfide nanoparticles were shown to have good catalytic activity for chemical reduction of p-nitroaniline in the presence of sodium borohydride.











Similar content being viewed by others
References
G. W. C. Li and H. Shan (2014). Highly efficient metal sulfide catalysts for selective dehydrogenation of Isobutane to Isobutene. ACS Catal 4, 1139–1143. https://doi.org/10.1021/cs5000944.
A. Gaiardo, B. Fabbri, V. Guidi, P. Bellutti, A. Giberti, S. Gherardi, L. Vanzetti, C. Malagù, and G. Zonta (2016). Metal sulfides as sensing materials for chemoresistive gas sensors. Sensors 16, 296. https://doi.org/10.3390/s16030296.
A. Hagfeldt and M. Grätzel (2000). Molecular photovoltaics. Acc. Chem. Res. 33, 269–277. https://doi.org/10.1021/ar980112j.
W. J. Parak, T. Pellegrino, and C. Plank (2005). Labelling of cells with quantum dots. Nanotechnology 16, R9–R25. https://doi.org/10.1088/0957-4484/16/2/R01.
C. H. Lai, M. Y. Lu, and L. J. Chen (2012). Metal sulfide nanostructures: synthesis, properties and applications in energy conversion and storage. J. Mater. Chem. 22, 19–30. https://doi.org/10.1039/C1JM13879K.
G. Govindasamya, P. Murugasenb, and S. Sagadevan (2017). Optical and electrical properties of chemical bath deposited cobalt sulphide thin films. Mater. Res. 20, 62–67. https://doi.org/10.1590/1980-5373-MR-2016-0441.
N. Rumale, S. Arbuj, G. Umarji, M. Shinde, U. Mulik, P. Joy, and D. Amalnerkar (2015). Tuning magnetic behavior of nanoscale cobalt sulfide and its nanocomposite with an engineering thermoplastic. J. Electron. Mater. 44, 2308–2311. https://doi.org/10.1007/s11664-015-3753-1.
R. Zahra, E. Pervaiz, M. Yang, O. Rabi, Z. Saleem, M. Ali, and S. Farrukh (2020). A review on nickel cobalt sulphide and their hybrids: earth abundant, pH stable electro-catalyst for hydrogen evolution reaction. Int. J. Hydrogen En. 45, 24518–24543. https://doi.org/10.1016/j.ijhydene.2020.06.236.
D. Ayodhya and G. Veerabhadram (2018). A review on recent advances in photodegradation of dyes using doped and heterojunction based semiconductor metal sulfide nanostructures for environmental protection. Mater. Today En. 9, 83–113. https://doi.org/10.1016/j.mtener.2018.05.007.
Y. Cui, C. Zhou, X. Li, Y. Gao, and J. Zhang (2017). High performance electrocatalysis for hydrogen evolution reaction using nickel-doped CoS2 nanostructures: experimental and DFT insights. Electrochim. Acta 228, 428–435. https://doi.org/10.1016/j.electacta.2017.01.103.
R. Akram, M. D. Khan, C. Zequine, X. Zhao, R. K. Guptha, M. Akhtar, J. Akhtar, M. A. Malik, N. Revaprasadu, and M. H. Bhatti (2020). Cobalt sulfide nanoparticles: synthesis, water splitting and supercapacitance studies. Mater. Sci. Semicond. Process. 109, 104925. https://doi.org/10.1016/j.mssp.2020.104925.
J. Kim, H. Jin, A. Oh, H. Baik, S. H. Joo, and K. Lee (2017). Synthesis of compositionally tunable, hollow mixed metal sulphide CoxNiySz octahedral nanocages and their composition-dependent electrocatalytic activities for oxygen evolution reaction. Nanoscale 9, 15397–15406. https://doi.org/10.1039/C7NR04327A.
H. Y. He (2017). Efficient interface-induced effect of novel reduced graphene oxide-CoS heteronanostructures in enhancing photocatalytic activities. Appl. Surf. Sci. 42, 260–267. https://doi.org/10.1016/j.apsusc.2016.10.128.
M. Xua, H. Niua, J. Huanga, J. Songa, C. Maoa, S. Zhanga, C. Zhub, and C. Chenc (2015). Facile synthesis of graphene-like Co3S4 nanosheet/Ag2S nanocomposite with enhanced performance in visible-light photocatalysis. Appl. Surf. Sci. 351, 374–378. https://doi.org/10.1016/j.apsusc.2015.05.158.
N. Pradhan, A. Pal, and T. Pal (2001). Catalytic reduction of aromatic nitro compounds by coinage metal nanoparticles. Langmuir 17, 1800–1802. https://doi.org/10.1021/la000862d.
S. Kundu, S. Lau, and H. Liang (2009). Shape-controlled catalysis by cetyltrimethylammonium bromide terminated gold nanospheres, nanorods and nanoprisms. J. Phys. Chem. C 113, 5157–5163. https://doi.org/10.1021/jp811331z.
I. Yoshiaki and K. Masa-ak (2000). Determination of p-phenylenediamine and related antioxidants in rubber boots by high performance liquid chromatography. Development of an analytical method for N-(1-methylheptyl)-N1-phenyl-p- phenylenediamine. J. Health Sci. 46, 467–473. https://doi.org/10.1248/jhs.46.467.
L. Hsiao-Shu and L. Yu-Wen (2009). Permeation of hair dye ingredients, p-phenylenediamine and aminophenol isomers, through protective gloves. Ann. Occup. Hyg. 53, 289–296. https://doi.org/10.1093/annhyg/mep009.
C.R. Barr, M. Pfaff (1971). Complexed p-phenylenediamine contain NG photographic element and development process therefor, USA patent-US3719492A
J. Liu, F. He, T. M. Gunn, D. Zhao, and C. B. Roberts (2009). Precise seed-mediated growth and size-controlled synthesis of palladium nanoparticles using a green chemistry approach. Langmuir 25, 7116–7128. https://doi.org/10.1021/la900228d.
S. J. Bao, C. M. Li, C. X. Guo, and Y. Qiao (2008). Biomolecule-assisted synthesis of cobalt sulfide nanowires for application in supercapacitors. J. Power Sour. 180, 676–681. https://doi.org/10.1016/j.jpowsour.2008.01.085.
G. Chen, W. Ma, D. Zhang, J. Zhu, and X. Liu (2013). Shape evolution and electrochemical properties of cobalt sulfide via a biomolecule-assisted solvothermal route. Solid State Sci. 17, 102–106. https://doi.org/10.1016/j.solidstatesciences.2012.08.025.
V. Reddy, S. R. Toarti, D. Rajeswari, S. S. M. Reddy, and B. Sanjay (2021). Facile and scalable preparation of bovine serum albumin stabilized cobalt sulfide nanostructures with various morphologies. Coll. Interf. Sci. Commun. 42, 100403. https://doi.org/10.1016/j.colcom.2021.100403.
K. R. Babu, J. J. Kumar, G. S. Bai, J. Singh, and V. Reddy (2021). Carboxymethyl cellulose stabilized lead sulfide nanocrystals: Synthesis, characterization and catalytic applications. Coll. Surf. A Physicochem. Eng. Aspects 620, 126572. https://doi.org/10.1016/j.colsurfa.2021.126572.
V. Reddy (2017). Green synthesis of platinum and gold nanoparticles and their self-assembled nanostructures. Chem. Sci. Trans. 6, 417–427. https://doi.org/10.7598/cst2017.1333.
J. Liu, J. Sutton, and C. B. Roberts (2007). Synthesis and extraction of monodisperse sodium carboxy methyl cellulose-stabilized platinum nanoparticles for the self-assembly of ordered arrays. J. Phys. Chem. C 111, 11566–11576. https://doi.org/10.1021/jp071967t.
R. Ramachandran, M. Saranya, C. Santhosh, V. Velmurugan, B. P. C. Raghupathy, S. K. Jeong, and A. N. Grace (2014). Co9S8 nanoflakes on graphene (Co9S8/G) nanocomposites for high performance supercapacitors. RSC Adv. 4, 21151–21162. https://doi.org/10.1039/C4RA01515K.
J. Tauc and A. Menth (1972). States in the gap. J. Nano-Cryst. Solids 569, 8–10. https://doi.org/10.1016/0022-3093(72)90194-9.
M. B. Muradov, O. O. Balayeva, A. A. Azizov, A. M. Maharramov, L. R. Qahramanli, G. M. Eyvazova, and Z. A. Aghamaliyev (2018). Synthesis and characterization of cobalt sulfide nanoparticles by sonochemical method. Infrared Phys. Technol. 89, 255–262. https://doi.org/10.1016/j.infrared.2018.01.014.
K. Y. Zou, Y. C. Liu, Y. F. Jiang, C. Y. Yu, M. L. Yue, and Z. X. Li (2017). Benzoate acid-dependent lattice dimension of Co-MOFs and MOF-derived CoS2@CNTs with tunable pore diameters for supercapacitors. Inorg. Chem. 56, 6184–6196. https://doi.org/10.1021/acs.inorgchem.7b00200.
H. Zhou and J. Hu (2017). Facile synthesis of multi-walled carbon nanotubes/Co9S8 composites with enhanced performances for sodium-ion battery. Mater. Lett. 195, 26–30. https://doi.org/10.1016/j.matlet.2017.02.004.
P. Cai, J. Huang, J. Chen, and Z. Wen (2017). Oxygen-incorporated amorphous cobalt sulfide porous nanocubes as high-activity electrocatalysts for the oxygen evolution reaction in an alkaline/neutral medium. Angew. Chem. Int. Ed. 56, 1–5. https://doi.org/10.1002/anie.201701280.
Z. Jiang, W. Lu, Z. Li, K. Hung Ho, X. Xu Li, and D. C. Jiao (2014). Synthesis of amorphous cobalt sulfide polyhedral nanocages for high performance supercapacitors. J. Mater. Chem. A 2, 8603–8606. https://doi.org/10.1039/C3TA14430E.
M. Kristl, B. Dojer, S. Gyergyek, and J. Kristl (2017). Synthesis of nickel and cobalt sulfide nanoparticles using a low cost sonochemical method. Heliyon 3, e00273. https://doi.org/10.1016/j.heliyon.2017.e00273.
B. Mullamuri, B. S. Diwakar, K. C. S. B. Kasturi, and V. Reddy (2017). Facile synthesis of bovine serum albumin conjugated low-dimensional ZnS nanocrystals. Int. J. Biol. Macromol. 101, 729–735. https://doi.org/10.1016/j.ijbiomac.2017.03.164.
C. Zhang, Y.-Y. Fu, X. Zhang, C. Yu, Y. Zhao, and S.-K. Sun (2015). BSA-directed synthesis of CuS nanoparticles as a biocompatible photothermal agent for tumor ablation in vivo. Dalton Trans. 44, 13112–13118. https://doi.org/10.1039/C5DT01467K.
V. Reddy, R. S. Torati, S. Oh, and C. G. Kim (2013). Biosynthesis of gold nanoparticles assisted by Sapindus mukorossi Gaertn fruit Pericarp and their catalytic application for the reduction of p-Nitroaniline. Indust. Eng. Chem. Res. 52, 556–564. https://doi.org/10.1021/ie302037c.
Acknowledgements
This work was supported by DST INSPIRE Faculty award of Dr. Venu Reddy (IFA13–ENG–70). Author JS acknowledges the DST INSPIRE Faculty award, New Delhi, and UGC New Delhi for financial support. Author JS also acknowledge Banaras Hindu University, Varanasi, for providing a seed grant under the Institute of Eminence (IoE) Dev. Scheme No. 6031.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Diwakar, B.S., Rajeswari, D., Singh, J. et al. Carboxymethyl Cellouse Stabilized Cobalt Sulfide Nanoparticles: Preparation, Characterization and Application. J Clust Sci 34, 2429–2439 (2023). https://doi.org/10.1007/s10876-022-02394-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-022-02394-4