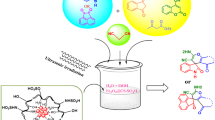
Abstract
Sulfoxides are versatile synthetic intermediates for the preparation of biological products. Therefore, there is a need for efficient methods to oxidize sulfides into sulfoxides. Such oxidation may be catalyzed by magnetic nanocatalysts due to their good stability, easy synthesis, high surface area, low toxicity and easy separation by magnetic forces. Here we prepared a nanocatalyst by immobilization of the chitosan–Schiff base complex on supramagnetic Fe3O4 nanoparticles. The chitosan–Schiff base complex has been previously prepared by functionalization of chitosan with 5-bromosalicylaldehyde and metalation with copper(II) acetate. The catalyst was characterized by Fourier transform infrared, powder X-ray diffraction, transmission electron microscope, scanning electron microscopy, energy-dispersive X-ray spectroscopy and thermogravimetric analysis. Results show that the Fe3O4 nanoparticles and nanocatalyst were spherical in shape with an average size of 20 nm. Upon the covalently anchoring of chitosan–Schiff base Cu complex on the magnetic Fe3O4 nanoparticles, the average size increased to 60 nm. The prepared Fe3O4–chitosan–Schiff base Cu complex catalyzed very efficiently the oxidation of sulfides to sulfoxides with 100 % selectivity in all cases under green reaction conditions and excellent yields. Additionally, ease of recovery and reusability up to four cycles without noticeable loss of catalytic activity make the present protocol beneficial from industrial and environmental viewpoint.


Similar content being viewed by others
References
Bisogno FR, Rioz-Martnez A, Rodguez C, Lavandera I, de-Gonzalo G, Torres Pazmico DE, Fraaije MW, Gotor V (2010) Oxidoreductases working together: concurrent obtaining of valuable derivatives by employing the PIKAT method. Chem Cat Chem 2:946–949. doi:10.1002/cctc.201000115
Buisson P, Quignard F (2002) Polysaccharides: natural polymeric supports for aqueous phase catalysts in the allylic substitution reaction. Aust J Chem 55:73–78. doi:10.1071/CH02004
Dai DY, Wang L, Chen Q, He MY (2014) Selective oxidation of sulfides to sulfoxides catalysed by deep eutectic solvent with H2O2. J Chem Res 38:183–185. doi:10.3184/174751914X13923144871332
Das R, Chakraborty D (2010) Cu(II)-catalyzed oxidation of sulfides. Tetrahedron Lett 51:6255–6258. doi:10.1016/j.tetlet.2010.09.081
Dreyer DR, Jia HP, Todd AD, Geng J, Bielawski CW (2011) Graphite oxide: a selective and highly efficient oxidant of thiols and sulfides. Org Biomol Chem 9:7292–7295. doi:10.1039/C1OB06102J
Ghorbani-Choghamarani A, Goudarziafshar H, Nikoorazm M, Yousefi S (2009) Efficient oxidation of sulfides to the sulfoxides using zirconium (IV) chloride, sodium nitrite and catalytic Amounts of bromide Ion as a novel oxidizing media. Lett Org Chem 6:335–339. doi:10.1002/chin.200946030
Gregori F, Nobili I, Bigi F, Maggi R, Predieri G, Sartori G (2008) Selective oxidation of sulfides to sulfoxides and sulfones using 30% aqueous hydrogen peroxide and silica-vanadia catalyst. J Mol Catal A: Chem 286:124–127. doi:10.1016/j.molcata.2008.02.004
Guo CC, Huang G, Zhang XB, Guo DC (2003) Catalysis of chitosan-supported iron tetraphenylporphyrin for aerobic oxidation of cyclohexane in absence of reductants and solvents. Appl Catal A Gen 247:261–267. doi:10.1016/S0926-860X(03)00108-X
Herdt AR, Kim BS, Taton TA (2006) Encapsulated magnetic nanoparticles as supports for proteins and recyclable biocatalysts. Bioconjug Chem 18:183–189. doi:10.1021/bc060215j
Iranpoor N, Mohajer D, Rezaeifard AR (2004) Rapid and highly chemoselective biomimetic oxidation of organosulfur compounds with tetrabutylammonium peroxymonosulfate in the presence of manganese meso-tetraphenylporphyrin and imidazole. Tetrahedron Lett 45:3811–3815. doi:10.1016/j.tetlet.2004.03.082
Jeong U, Teng X, Wang Y, Yang H, Xia Y (2007) Superparamagnetic colloids: controlled synthesis and niche applications. Adv Mater 19:33–60. doi:10.1002/adma.200600674
Kasaai MR (2009) Various methods for determination of the degree of N-acetylation of chitin and chitosan: a review. J Agric Food Chem 57:1667–1676. doi:10.1021/jf803001m
Khurana JM, Panda AK, Ray A, Gogia A (1996) Rapid oxidation of sulfides and sulfoxides with sodium hypochlorite. Org Prep Proced Int 28:234–236. doi:10.1080/00304949609356529
Kumar KS, Kumar VB, Paik P (2013) Recent advancement in functional core-shell nanoparticles of polymers: synthesis, physical properties, and applications in medical biotechnology. J Nanopart. doi:10.1155/2013/672059
Lakouraj MM, Tajbakhsh M, Shirini F, Tamami MVA (2005) HIO3 in the presence of wet SiO2: a mild and efficient reagent for selective oxidation of sulfides to sulfoxides under solvent-free conditions. Synth Commun 35:775–784. doi:10.1081/SCC-200050940
Laurent S, Forge D, Port M, Roch A, Robic C, Elst LV, Muller RN (2008) Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev 108:2064–2110. doi:10.1021/cr068445e
Lertworasirikul A, Tsue SI, Noguchi K, Okuyama K, Ogawa K (2003) Two different molecular conformations found in chitosan type II salts. Carbohydr Res 338:1229–1233. doi:10.1016/S0008-6215(03)00145-9
Polshettiwar V, Baruwati B, Varma RS (2009) Magnetic nanoparticle-supported glutathione: a conceptually sustainable organocatalyst. Chem Commun. doi:10.1039/B900784A
Safari J, Javadian L (2014) Chitosan decorated Fe3O4 nanoparticles as a magnetic catalyst in the synthesis of phenytoin derivatives. RSC Adv 4:48973–48979. doi:10.1039/C4RA06618A
Shaabani A, Rezayan AH (2007) Silica sulfuric acid promoted selective oxidation of sulfides to sulfoxides or sulfones in the presence of aqueous H2O2. Catal Commun 8:1112–1116. doi:10.1016/j.catcom.2006.10.033
Sharma SP, Suryanarayana MVS, Nigam AK, Chauhan AS, Tomar LNS (2009) [PANI/ZnO] composite: catalyst for solvent-free selective oxidation of sulfides. Catal Commun 10:905–912. doi:10.1016/j.catcom.2008.12.021
Sun C, Lee JSH, Zhang M (2008) Magnetic nanoparticles in MR imaging and drug delivery. Adv Drug Deliv Rev 60:1252–1265. doi:10.1016/j.addr.2008.03.018
Varma AJ, Deshpande SV, Kennedy JF (2004) Metal complexation by chitosan and its derivatives: a review. Carbohydr Polym 55:77–93. doi:10.1016/j.carbpol.2003.08.005
Vincent T, Guibal E (2002) Chitosan-supported palladium catalyst. 1. Synthesis procedure. Ind Eng Res Chem 41:5158–5164. doi:10.1021/ie0201462
Xie HY, Zhen R, Wang B, Feng YJ, Chen P, Hao J (2010) Fe3O4/Au core/shell nanoparticles modified with Ni2+-nitrilotriacetic acid specific to histidine-tagged proteins. J Phys Chem C 114:4825–4830. doi:10.1021/jp910753f
Yu B, Liu AH, He LN, Li B, Diao ZF, Li YN (2012) Catalyst-free approach for solvent-dependent selective oxidation of organic sulphides with oxone. Green Chem 14:957–962. doi:10.1039/C2GC00027J
Acknowledgments
The authors gratefully acknowledge the funding support received from the Ilam University, Ilam, Iran, on this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naghipour, A., Fakhri, A. Efficient oxidation of sulfides into sulfoxides catalyzed by a chitosan–Schiff base complex of Cu(II) supported on supramagnetic Fe3O4 nanoparticles. Environ Chem Lett 14, 207–213 (2016). https://doi.org/10.1007/s10311-015-0545-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-015-0545-z