Abstract
This paper reports the preparation of copper(I) oxide nanoparticles deposited on yttrium oxide and copper(II) oxide in the presence of acerola and white willow extracts. Through the use of natural compounds, it was possible to modify the surface of the Y2O3 and CuO carriers allowing Cu2O to be deposited to a greater extent, thus improving the antibacterial properties of the materials. Cu2O nanoparticles, by being deposited on a carrier, enable an increase in the contact surface of the nanoparticles with microorganisms, which react to form reactive oxygen species. Cu2O nanoparticles with sizes of about 38 nm and 76 nm were obtained for Y2O3- and CuO-deposited nanoparticles, respectively. The Gram-negative bacteria Escherichia coli shown a greater sensitivity to the degree of inhibition compared to Staphylococcus Aureus already at a concentration of 250 mg/L. For almost all materials, the inhibition level remained above 50% after 48 h. Analysis of the effect of the antimicrobial properties of the materials against Candida albicans fungus shown high activity which was obtained only at the highest concentrations of 8000 mg/L, for which the degree of growth inhibition was 100% also after 48 h for both Y2O3–Cu2O and CuO–Cu2O.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The combination of nanotechnology and biotechnology has enabled the development of the new discipline of nanobiotechnology, one of the most promising technologies of the twenty-first century [1, 2]. Nanobiotechnology plays an important role in the development of medicine and pharmacy and has found applications in obtaining numerous useful devices, technologies and drugs [3,4,5,6].
Among inorganic nanoparticles used in industry, nanoparticles of metals and metal oxides are the most important [7, 8]. Inorganic nanoparticles are a promising class of antimicrobial agents as an alternative to antibiotics [9, 10]. In contrast, nanoparticles target most bacteria and fungi, exhibiting a broad spectrum of activity [11]. Among nanoparticles with antimicrobial properties, silver and copper nanoparticles remain the most significant. They show a broad spectrum of activity and high efficiency in degrading bacteria, fungi, and viruses [12]. Despite the high biocidal activity of metal nanoparticles, cheaper materials with high stability and prolonged activity are being sought. An alternative to metal nanoparticles is copper(I) oxide. Copper and its complexes have been used as fungicides, bactericides and algicides [13].
Cu2O nanoparticles have attracted interest due to their non-toxicity, good mobility, high availability, and low cost [14, 15]. They are used as antimicrobial agent, photocatalyst, in solar cells and gas sensor [16]. Cu2O is a p-type semiconductor with an energy gap of 2.2 eV with a high antimicrobial activity and low cost [17]. Due to these practical properties, Cu2O nanoparticles have been widely studied in various application areas such as batteries, catalysis, gas sensor, biosensors, magnetic storage devices, medicine, antifouling coatings, photovoltaics, and photocatalytic degradation of most organic pollutants [18, 19].
An increase in the active surface area of nanoparticles including Cu2O enhances the antimicrobial activity of the materials [20]. An alternative approach is to disperse and deposit copper(I) oxide nanoparticles on a carrier such as yttrium oxide [21] and copper(II) oxide [22], which are classified as bioactive oxides. This approach increases the active surface area of the active substance.
In biomedicine, Y2O3 and CuO nanoparticles are showing a variety of applications such as drug delivery systems, gene therapy, protein, and tissue identification [23,24,25]. The unique optoelectronic, mechanical, and physicochemical properties of CuO and Y2O3 NPs can provide a multifunctional platform and be used as drug carriers and in diagnostics. Setua et al. report that Y2O3 nanocrystals doped with europium (Eu3+) and gadolinium (Gd3+) ions and conjugated with a targeting ligand (folic acid) have been used for bimodal fluorescence and magnetic imaging of cancer cells [26]. However, due to the limited information on how the bonding process between metal oxides and the nanoparticles combined with it, it is necessary to understand the biological interactions and biocompatibility of the materials [27].
It is suggested that the combination of copper(I) oxide nanoparticles with Y2O3 or CuO oxide will enhance the Cu2O activity, by developing the surface of the material, and will gradually release Cu2+ ions which will prolong the performance of the material [28]. At the same time, it will secure the gradual oxidation of Cu2+ ions to CuO, which are more stable but also less reactive. The process of obtaining Cu2O/CuO composite deposited on fabric was carried out. The in-situ synthesis of CuO/Cu2O NPs involved the adsorption of Cu2+ ions by the introduced amino groups of the sol–gel coating and the reduction of Cu2+ ions by NaBH4. The antimicrobial activity against Gram-negative Escherichia coli, Klebsiella pneumoniae, Gram-positive Staphylococcus aureus bacteria and Candida albicans yeast was strongly dependent on the copper content. In addition to the excellent antimicrobial activity, a controlled release of Cu2+ ions from the fabrics into the saline solution was obtained [29, 30]. Regmi et al. investigated an uncomplicated method to obtain Cu2O NPs on the surface of SiO2 NPs. It was confirmed that Cu2O deposition on SiO2 NPs is an efficient inorganic support with tremendous chemical and thermal stability, decent biocompatibility, high specific surface area [31].
According to our best knowledge, there are no studies accessible on the functionality of Cu2O combinations with Y2O3 nanoparticles and verification of their antimicrobial properties. Compounds contained in acerola and white willow extracts, firstly, increase the contact surface area of the Y2O3 carriers and enhance the surface defect of the nanoparticles. In addition, the carriers modified in this way increase the antimicrobial activity of the Cu2O nanoparticles by interacting synergistically with them. The aim of this study was to investigate the effect of carrier type on the antimicrobial properties and stability of Cu2O nanoparticles. In this study, it is hypothesised that the type of extract used and its form (liquid/solid) can affect the specific surface area and the occurrence of functional groups, which will influence the efficiency of the carrier nanoparticles to combine with Cu2O and can affect the antimicrobial properties of the final materials. This study may provide a basis for a better understanding of the environmental behaviour and biological biocidal properties of Y2O3–Cu2O and CuO–Cu2O NPs.
Experimental
Materials
Aqueous solutions of yttrium nitrate (Sigma Aldrich), copper(II) acetate (Merck), copper(II) sulphate (Sigma Aldrich), glucose (Sigma Aldrich) and sodium hydroxide (Sigma Aldrich) were used to obtain dioxide systems. Surface functionalization of Y2O3 and CuO oxides was carried out using a range of liquid and solid extracts (Table 1).
Selection of Extracts for Surface Modification of Y2O3 and CuO Nanoparticles
Extracts from selected plants were used to obtain yttrium oxide and copper(II) oxide. The following extracts in liquid form were used: pineapple (Ananas comosus), peppermint (Mentha piperita), garden strawberry leaves (Fragaria ananassa), acerola (Malpighia emarginata) and extracts in solid form: white willow (Salix alba), prickly pear (Opuntia ficus-indica), pomegranate peel (Punica granatum), tangerine (Citrus tangerina). The presence of two forms of extracts was to provide, on the one hand, an increased amount of natural active compounds based on alkaloids, terpenes, flavonoids and polyphenols affecting the metal ion reduction reactions and stabilisation of the forming particles and, on the other hand, to provide a wide spectrum of functional groups modifying the surface of the materials.
Accordingly, the extracts were selected as a result of analysis of the content of compounds from the polyphenol and flavonoids groups. Higher content of components of natural origin enabled higher degree of functionalization of metal oxides surface. Preliminary evaluation of the extracts was carried out by determining the total content of polyphenols, flavonoids and additionally by testing the antioxidant potential against DPPH radical.
Total Polyphenol Content
The total polyphenols content was determined by the Folin–Ciocâlteu method. This method is based on colorimetric reactions and uses UV–Vis spectrophotometry. As a reference substance, gallic acid is used. The result is a statement of the sum of the concentration of phenolic hydroxyl groups present in the extract. First, a calibration curve for gallic acid was prepared. Calibration solutions of gallic acid with concentrations ranging from 10 to 100 µg/ml were made. An 7.5% aqueous sodium carbonate solution was also prepared and the Folin-Ciocâlteu reagent was diluted 1:9 (v/v) with water and stored in a dark glass bottle. The analysis was carried out by mixing 0.5 ml of a standard solution of known gallic acid concentration or 0.5 ml of deionised water (reference sample) with 2.5 ml of Folin–Ciocâlteu reagent and 2 ml of sodium carbonate solution in test tubes. After mixing, the tubes were kept in the darkroom for 30 min. After this time, the absorbance of the coloured solutions at λ = 765 nm was measured. The results are presented in Table 1.
Flavonoid Content
The determination of total flavonoid content was examined spectrophotometrically. To a 10 ml flask, 0.1 ml of extract, 3 ml of 2% AlCl3 solution were added and the total was made up with methanol. The standard solution was prepared by adding 3 ml of 2% AlCl3 solution and 7 ml of methanol. After 25 min, the absorbance of the test solutions was measured at λ = 430 nm against the blank solution. The flavonoid content was calculated in terms of quercetin content. For this purpose, a standard curve was prepared by preparing a solution of 0.050 mg/ml quercetin, which was diluted to obtain concentrations of 1, 5, 10, 15, 20 and 25 µg/ml. The results were expressed as the amount of flavonoids mg/ml of the extract.
Antioxidative Potential of Materials Against DPPH Radical
Free radical scavenging was determined using the reagent DPPH (1,1-diphenyl-2-picrylhydrazyl). Reduction of stable violet DPPH to yellow diphenylpicrylhydrazine allows calculation of the percentage of inhibition. The absorbance of the DPPH solution was read at λ = 517 nm after 30 min. The antioxidant activity of the extracts was expressed as the percentage reduction of the DPPH radical with the extract relative to the sample without antioxidant:
where I—percentage inhibition of DPPH, Ac—absorbance of the control sample, As—absorbance of the extract samples.
Method of Synthesis of Extract-Y2O3 or Extract-CuO
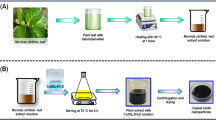
As a first step, metal oxide was obtained as a base for the deposition of copper(I) oxide, ensuring its stability and limiting the oxidation of copper from Cu+ to Cu2+. Yttrium oxide was obtained by mixing 10 ml of 0.012 M Y(NO3)3 with 22.4 ml of 0.08 M NaOH. To prepare the modified extract-Y2O3, 2 ml of liquid extract or 0.5 g of solid extract was added before adding NaOH. The suspension was ultrasonicated for 2 min to disperse the material and then the sample was microwaved for 20 min at 200 °C. In order to obtain yttrium hydroxide, the material was filtered, washed with deionised water, dried at 70 °C for 24 h and then calcined at 700 °C for 2 h to dehydrate Y(OH)3 and obtain Y2O3 and to remove carbon residues from the extracts.
The preparation of CuO nanoparticles was carried out in an analogous manner using copper(II) sulphate as an oxide precursor.
Synthesis of Cu2O Nanoparticles
After obtaining the bases in the form of yttrium oxide and copper(II) oxide particles, the process of preparation with simultaneous deposition of copper(I) oxide nanoparticles on their surface was carried out. For this purpose, 50 ml of copper(II) acetate solution was added to 500 mg of Y2O3 or CuO and stirred for 60 min in order to absorb a maximum amount of Cu2+ ions on the surface. After this time 10 ml of a 10% solution of glucose was added to reduce the copper(II) ions to copper(I) and the suspension was stirred for a further 60 min. After this time the material was filtered and washed. After drying at 105 °C, Cu2O nanoparticles deposited on yttrium or copper(II) oxide were obtained.
Instrumental Analysis
The properties of the obtained dioxide compounds were investigated by XRD, from which the composition of the materials, the size of the crystallites, and Rietveld analysis were determined, allowing quantitative characterisation of the products. The particle size of the materials was determined by DLS analyses. FTIR analysis made it possible to describe the differences in surface functionalization of Y2O3 and CuO nanoparticles after modification with extracts. SEM–EDS analysis made it possible to determine the deposition distribution of Cu2O nanoparticles on the surface of the base oxides and to assess the morphology and shape of the nanoparticle materials. The degree of Cu2+ ion leaching over time was investigated using a copper ion-selective electrode, allowing detection of copper ions in the range of 1 to 1000 mg/l.
Antimicrobial Properties
The antimicrobial properties of Y2O3-Cu2O and CuO-Cu2O nanoparticles were tested against both gram-positive and gram-negative bacteria: Staphylococcus aureus and Escherichia coli (American Type Culture Collection), as well as against pathogenic fungus Candida albicans (National Collection of Yeast Cultures,). All microorganisms were pre-cultured from bank cultures and then main liquid cultures were prepared (E. coli—Lenox Broth—LB medium, S. aureus—Brain Heart Infusion Broth—BHI medium, C. albicans—Yeast extract, Peptone, Dextrose—YPD medium). Experiments were performed with the use of sterile 96-well microdilution plate. 50 µl and 100 ul of proper growth medium was added to each well in columns 2–6 and columns 7–8, respectively. To all wells in first column (starting from A1 till H1), the highest concentration of tested material was added (100 µl, 8000 mg/l). Then, following serial dilution method, tested materials were diluted (by transferring 50 µl of suspension from one well to the next well) till the final concentration 250 mg/l in each row. Wells in column 7 were inoculated with 50 µl of liquid culture of microorganisms and were standing as a negative control (microorganisms without any compounds). To the last column (8) only medium was added as a medium sterility control (medium without any microorganisms). Plates were incubated at 37 °C for 24 h and for 72 h for E. coli, S. aureus and for C. albicans, respectively. After the incubation, 10 µl of each well solution was evenly distributed on the surface of proper solid media on Petri dish (BHI agar—S. aureus, MacConkey agar—E. coli, Sabouraud agar—C. albicans) with the use of smoothing pad. Then, all of the inoculated plates were placed in the incubator for 24 and 48 h and the percentage value of growth inhibition was calculated for each sample after both 24 and 48 h. Data is shown as a mean value ± SD from triplicate samples. The results are presented as bar graphs and divided according to the microorganisms tested and the incubation time.
Results
Characteristics of Obtained Materials Y2O3 and CuO
Characteristics of Extracts
The results of the analysis of active components in the tested extracts are presented in Table 1. The highest content of polyphenols and flavonoids was recorded for extracts from peppermint (Mentha piperita), acerola (Malpighia emarginata), white willow (Salix alba) and prickly pear (Opuntia ficus-indica). In each of the extracts, one liquid and one solid extract were selected for further studies. Peppermint extract was characterised by lower polyphenolic content and lower ability to reduce DPPH radical, therefore acerola extract was selected for further studies. Comparing the pear extract with the white willow extract, the second one had higher flavonoid content and higher efficiency of DPPH radical reduction, and hence the white willow extract was used to obtain Y2O3 and CuO oxides. The acerola content was obtained similarly to the study by Rezende et al. which compared the content of active compounds in pulp extract and residue extract. Total polyphenol content was over 10,000 µg/ml independently of the pulp source [32].
XRD Analysis
The materials after calcination were subjected to XRD analysis. Diffractograms of nanoparticles of single oxides and dioxides are shown in Fig. 1. Nanoparticles of pure yttrium, copper(II) and copper(I) oxides did not contain any impurities and all peaks characteristic for standard materials were confirmed. The Y2O3 nanoparticles had a cubic structure with a crystal structure of I a -3 [33]. The use of extracts in the yttrium hydroxide precipitation process resulted in particles with increased elementary cell size (Table 2).
Deposition of Cu2O nanoparticles on the Y2O3 surface resulted in covering the crystalline surfaces of yttrium oxide; however, the material showed a significant increase in crystallinity as confirmed by nucleation of Cu2O on the yttrium oxide surface and gradual growth of Cu2O crystals. Cu2O nanoparticles obtained separately as well as on oxide surfaces were characterised by a cubic structure with an average elemental cell size of 4.26 Å. The copper(II) oxide nanoparticles were formed in a monoclinic form with the crystallographic structure P n -3 [34, 35]. Due to the different distribution of elementary cells, the nucleation and formation of Cu2O nanoparticles proceeded differently, hence the presence of two phases: CuO and Cu2O.
Effect of Extract Type on the Size of Y2O3 and CuO Nanoparticles
Table 3 shows a comparison of the particle and crystallite size results of the obtained Y2O3, CuO, Cu2O nanoparticles and their combinations. The presence of extracts from both acerola and willow resulted in nanoparticles with smaller crystallite sizes. The addition of Cu2O nanoparticles in the second stage caused a slight further increase in the crystallites of the base Y2O3 and CuO oxides. At the same time, the addition of the extracts caused a decrease in the crystallinity of the materials resulting in the formation of less regular crystals.
SEM Imaging of Nanoparticle Materials
SEM analysis confirmed the formation of spherical yttrium particles with a diameter of about 1 um also in the presence of acerola and willow extract, but the addition of the extract results in smaller particle sizes (Fig. 2). Copper(I) oxide nanoparticles with an average size of about 35–45 nm are deposited on the yttrium oxide surface (Table 4). Copper(II) oxide without the addition of extract caused the formation of star-shaped particles on the surface of which Cu2O nanoparticles were deposited. The addition of acerola extract in the form made it possible to obtain regular particles. The particles formed by the addition of willow extract in solid form have similar size and appearance to the CuO-AC nanoparticles but are characterised by lower regularity.
SEM microphotographs of nanoparticles: A Y2O3–Cu2O, B Y2O3-AC-Cu2O, C Y2O3-WW-Cu2O, D size distribution of Cu2O for Y2O3–Cu2O, E size distribution of Cu2O for Y2O3-AC-Cu2O, F size distribution of Cu2O for Y2O3-WW-Cu2O, G size distribution of Y2O3 for Y2O3–Cu2O, H size distribution of Y2O3 for Y2O3-AC-Cu2O, I size distribution of Y2O3 for Y2O3-WW-Cu2O, J CuO–Cu2O, K CuO-AC-Cu2O, L CuO-WW-Cu2O, M size distribution of Cu2O for CuO–Cu2O, N size distribution of Cu2O for CuO-AC-Cu2O, O size distribution of Cu2O for CuO-WW-Cu2O, P size distribution of CuO for CuO–Cu2O, R size distribution of CuO for CuO-AC-Cu2O, S size distribution of CuO for CuO-WW-Cu2O
The spherical shape of yttrium oxide is consistent with results obtained in the literature [36, 37]. Nunes et al. based on the nitric acid and hydrochloric acid dissolution method, it was possible to produce yttrium oxide nanoparticles with a size of approximately 200–600 nm, and the nanoparticles were characterised by increased polydispersity [38]. In the case of obtaining CuO nanoparticles, the method used and the conditions of synthesis have a major impact on the shape and size of the particles obtained [39,40,41]. Bhosale et al. depending on the choice of acid (cinnamic, oxalic, adipic, fumaric and succinic acids) obtained CuO nanoparticles with the shape of rods, wires, belts [42].
BET Analysis
A low temperature nitrogen adsorption (BET analysis) was performed to determine the specific surface area of the adsorbent. The modification of the oxides with a solid willow extract allowed to obtain materials with the most developed surface area, compared to the use of liquid acerola extract and in the absence of modification. The presence of additional solid particles present in the solid willow extract during the calcination process enables additional surface development.
In yttrium oxide nanoparticles, the presence of nanopores and the presence of mesopores were confirmed, while in the case of copper(II) oxide analysis, the presence of nanopores was observed, but their proportion is lower compared to Y2O3. Hysteresis loop analysis of Y2O3-Cu2O nanoparticles shows the formation of H3 loop indicating the presence of slitshaped pore. For CuO-Cu2O systems an H4 hysteresis loop was obtained, which is characteristic for wedgeshaped pores (Fig. 3).
FTIR Analysis
The effect of the presence of extracts on the surface functionalisation of the oxide materials was investigated by FTIR analysis (Fig. 4). For both types of oxides, no bands were observed at about 3400 cm−1 associated with the presence of water.
For yttrium oxide-based materials, bands at 602 cm−1 corresponding to Me–O stretch bonds were observed. The bands at 1593 cm−1 represents the water and the hydroxyl stretches, respectively and the band at 653.51 cm−1 represents the Y–O stretch and the results were in correlation with an earlier report [43]. The absorption peak at 1377 cm−1 was assigned to vibration modes of nitrate anions [44]. Frequencies between 1074 and 1022 cm−1 are assigned for the characteristic asymmetric stretching of Y–O–Y [45]. The peak at 870, 818 cm−1 are responsible for the presence of trace of Y–OH [46].
Antimicrobial Properties
Leaching of Cu2+ from Nanoparticles
The copper ion leaching process was carried out for 120 min. In all materials the highest concentrations of copper ions were observed after the first minute, which is related to their desorption from the surface of the materials, with the highest Cu2+ contents observed for Y2O3-Cu2O materials (Fig. 5). In materials containing CuO the rate of copper ion leaching is increased but remains constant which may affect the bioactivity of the materials [47]. After 5 min the equilibrium between the rate of desorption and adsorption of copper ions is reached and with time a slight increase in ion concentration is observed, which may be due to the gradual process of Cu2O degradation and transition to the ionic form.
Efficacy of Microbial Growth Inhibition
Based on the study, E. coli is most susceptible to the materials (Fig. 6). The Gram-negative bacteria E. coli shows a greater sensitivity to the degree of inhibition compared to S. Aureus already at a concentration of 250 mg/l. The materials with the highest antimicrobial activity against E. coli are based on Y2O3–Cu2O, both for formulations without and with the addition of acerola extract, while in the case of CuO-based martials, CuO–WW–Cu2O shows the best growth inhibitory properties. For almost all materials, the inhibition level remains above 50% after 48 h. Mani et al. analysing the antibacterial and antifungal activity of the synthesised CuO nanoparticles showed the highest activity against P. aeruginosa, E. coli and V. cholerae, confirming the high efficacy of copper oxide nanoparticles against bacteria. The authors suggested that one of the main mechanisms affecting antibacterial activity is the formation of reactive oxygen species (ROS), which disrupt the cell membrane through protein oxidation and lipid peroxidation, so that eventually NPs degrade DNA in bacterial cells [48]. Nguyen and Trinh determined the activity of Cu2O NPs, considering both the method of obtaining the nanoparticles and which form of copper would exhibit the highest bioactivity. Cu2O nanoparticles were synthesised by reducing CuSO4 with glucose in the presence of polyvinyl alcohol as a stabilising and growth-limiting agent. Different synthetic procedures were used. The first procedure was carried out in one step at 60 °C for 15 min under continuous stirring. The second method was carried out in two stages, in which initially half of the reagents were stirred rapidly at high temperature, to slowly add the rest of the components at low temperature in the second stage. Comparing particle distributions revealed that the first method yielded smaller nanoparticles, while the second procedure obtained larger but more uniform NPs. Next, the authors analysed the antibacterial activity of Cu2O against E. coli. Under all conditions tested, Cu2O NP showed significantly higher inactivation efficiency compared to CuSO4, in which copper was present in ionic form. Increasing bactericidal activity was shown sequentially for CuSO4, CuO and Cu2O. This is related to the ability of CuO and Cu2O NPs to adhere and penetrate the cell membrane to release copper ions inside the cell [49].
In case of S. aureus, the Y2O3–WW–Cu2O material showed the highest activity, for which the inhibition percentage after 48 h was more than 90% already for a concentration of 2000 mg/l. In general, for Y2O3–Cu2O materials the activity against S. aureus is much higher compared to CuO–Cu2O, among which CuO–AC–Cu2O presents the most effective antimicrobial activity.
Analysis of the effect of the antimicrobial properties of the materials on the fungus C. albicans shows high activity only at the highest concentrations of 8000 mg/l, for which the degree of growth inhibition is 100% also after 48 h (for Y2O3–Cu2O, Y2O3–AC–Cu2O and CuO–AC–Cu2O materials). An exponential decrease in the effectiveness of the materials is observed at subsequent dilutions. However, higher activity was observed for CuO-based compounds compared to Y2O3. This may be due to the higher leaching of copper(II) ions. Woźniak-Budych et al., investigated the effects of copper ion release kinetics from stabilised Cu2O nanoparticles in four different biological fluids: simulated saliva, human blood plasma, gastric fluid, and intestinal fluid. At particle sizes between 150 and 700 nm, the highest copper ion content was observed for Cu2O NPs in simulated gastric fluid (45–47 μg/ml). The authors observed that Cu2O nanoparticles in contact with biological fluids formed aggregates and gradually released copper ions, which increased the cytotoxicity of Cu2O NPs [50].
Despite ongoing research, the mechanism of action of inorganic nanoparticles on microorganisms is not clearly described. Among the methods of action of copper oxide nanoparticles, the release of copper ions into the environment, including the interior of microorganisms, is of great importance. The occurrence of copper in the form of Cu(II) and Cu(I) ions enables the generation of reactive oxygen and nitrogen species, which in turn have a destructive effect on bacterial and fungal cells. Hasheminya et al. confirmed the effectiveness of copper oxides against E. coli and S. aureus, among others, in which the release of copper ions was one of the main cell-destroying mechanisms. Cu2+ ions easily penetrated the cell membrane, destroying cells from within [51].
Copper compounds, especially copper(I) compounds can generate DNA damaging ROS i.e., Hydrogen peroxide (H2O2), superoxide (O2−), hydroxyl radical (–OH) and singlet oxygen (1O2). The copper(I) oxide among them remains stable under standard conditions and therefore shows higher activity compared to CuO [26]. On the surface of Cu2O nanoparticles, free radicals are gradually generated, which interfere with oxidation–reduction processes in the cell. Deposition of Cu2O nanoparticles on a carrier allows to increase the active surface area, thus improving their bioactivity [16]. The high antimicrobial activity of Cu2O was confirmed by Ren et al. The authors confirmed that both octahedral and cubic nanoparticles can effectively inhibit the growth of E. coli [52]. Also, Angelé-Martínez et al. obtained bioactive Cu2O nanoparticles that already at a concentration of 25 μg/ml caused an 85% decrease in E. coli cell survival [53].
Conclusion
In this paper the process of preparation of copper(I) oxide nanoparticles deposited on yttrium oxide and copper(II) oxide nanoparticles is presented. Y2O3 and CuO base nanoparticles were prepared in the presence of liquid acerola extract and solid white willow extract. The presence of the extracts in different forms caused the metal oxide surfaces to be functionalized with additional functional groups, among others, and the surface area of the extracts was developed and the particle sizes were reduced.
Copper(I) oxide nanoparticles deposited on the surface of metal oxides showed higher antimicrobial activity against bacteria and fungi. As a result of the deposition of Cu2O nanoparticles, leaching of Cu2+ ions was observed to a lower extent in the case of CuO–Cu2O systems and to a higher extent in Y2O3–Cu2O materials. This is confirmed by microbiological studies in which Y2O3–Cu2O materials showed higher activity. A dual effect of Cu2O nanoparticles is suggested. Firstly, Cu2+ ions are released over time, showing bioactive effects, and secondly, Cu2O nanoparticles cause gradual oxidation to CuO over time, thus acting on pathogen cells in a redox process, disrupting their economy and destabilising their system. The continuing interest in nanoparticles and their subsequent modifications undoubtedly indicate the need for further research on new systems showing better properties, wider application possibilities and lower production costs. The materials based on copper and yttrium obtained in this work meet these requirements and further research on this subject will be continued.
References
K. Agrawal, V. K. Gupta, and P. Verma (2021). Crit. Rev. Microbiol. 48, 397. https://doi.org/10.1080/1040841X.2021.1977779.
H. E. Emam and H. B. Ahmed (2021). Int. J. Biol. Macromol. 170, 688. https://doi.org/10.1016/J.IJBIOMAC.2020.12.151.
B. Du Duy, D. Van Phu, L. Anh Quoc, and Hien N. Quoc (2017). J Nanopart. https://doi.org/10.1155/2017/7056864.
R. Farsi (2022). J Pharm Res Int. https://doi.org/10.9734/JPRI/2022/V34I8A35476.
S. Thanigaivel, A. K. Priya, L. Gnanasekaran, T. K. A. Hoang, S. Rajendran, and M. Soto-Moscoso (2022). Sustain. Energy Technol. Assess. 53, 102484. https://doi.org/10.1016/J.SETA.2022.102484.
B. De and T. K. Goswami (2022). Biotechnol Zero Waste. https://doi.org/10.1002/9783527832064.CH25.
K. Parangusan, V. Subramanium, L. Sundarabharathi, K. Kannan, and D. Radhika (2021). Res Sq. https://doi.org/10.21203/RS.3.RS-385905/V1.
C. A. Roque-Borda, P. BentodaSilva, M. C. Rodrigues, L. D. diFilippo, J. L. Duarte, M. Chorilli, et al. (2022). Eur. J. Med. Chem. 241, 114640. https://doi.org/10.1016/J.EJMECH.2022.114640.
V. S. Gondil, K. Harjai, and S. Chhibber (2020). Int. J. Antimicrob. Agents 55, 105844. https://doi.org/10.1016/J.IJANTIMICAG.2019.11.001.
R. G. Otto, E. van Gorp, W. Kloezen, J. Meletiadis, S. van den Berg, and J. W. Mouton (2019). Int. J. Antimicrob. Agents 53, 34. https://doi.org/10.1016/J.IJANTIMICAG.2018.09.003.
W. E. Org, B. Han, Y. Yang, H. Deng, Y. Chen, and C. Yang (2018). Int. J. Electrochem. Sci. 13, 5681. https://doi.org/10.20964/2018.06.66.
P. Kamaraj, M. Sridharan, and J. Arockiaselvi, Malaya J. Matematik. https://doi.org/10.26637/MJM0S20/0644.
X. Cheng, Y. Zhao, Z. Qian, J. Wu, J. Dong, Z. Ma, et al. (2021). Mater. Sci. Eng. A 824, 141867. https://doi.org/10.1016/J.MSEA.2021.141867.
H. Jung, S. Y. Lee, C. W. Lee, M. K. Cho, D. H. Won, C. Kim, et al. (2019). J. Am. Chem. Soc. 141, 4624. https://doi.org/10.1021/JACS.8B11237/SUPPL_FILE/JA8B11237_SI_001.PDF.
M. Mallik, S. Monia, M. Gupta, A. Ghosh, M. P. Toppo, and H. Roy (2020). J. Alloys Compd. 829, 154623. https://doi.org/10.1016/J.JALLCOM.2020.154623.
J. P. Prajapati, D. Das, S. Katlakunta, N. Maramu, V. Ranjan, and S. Mallick (2021). Inorg. Chim. Acta 515, 120069. https://doi.org/10.1016/J.ICA.2020.120069.
L. Dou, X. Zhang, M. M. Zangeneh, and Y. Zhang (2021). Bioorg. Chem. 106, 104468. https://doi.org/10.1016/J.BIOORG.2020.104468.
X. Yu, X. Cao, L. Yue, J. Zhao, F. Chen, Z. Wang, and B. Xing (2020). Sci. Total Environ. 732, 139276. https://doi.org/10.1016/j.scitotenv.2020.139276.
S. K. Pradhan, V. Pareek, J. Panwar, and S. Gupta (2019). J. Water Process Eng. 32, 100917. https://doi.org/10.1016/J.JWPE.2019.100917.
K. Zodrow, L. Brunet, S. Mahendra, D. Li, A. Zhang, Q. Li, et al. (2009). Water Res. 43, 715. https://doi.org/10.1016/J.WATRES.2008.11.014.
G. M. Abu-Taweel, H. M. Albetran, M. G. Al-Mutary, M. Ahmad, and I. M. Low (2021). Toxicol. Rep. 8, 1121. https://doi.org/10.1016/J.TOXREP.2021.05.014.
M. Y. Chong, A. Numan, C.-W. Liew, K. Ramesh, and S. Ramesh (2017). J. Appl. Polym. Sci. 134, 44636. https://doi.org/10.1002/APP.44636.
G. Rajakumar, L. Mao, T. Bao, W. Wen, S. Wang, T. Gomathi, et al. (2021). Appl. Sci. 11, 2172. https://doi.org/10.3390/APP11052172.
N. Pandiyan, B. Murugesan, M. Arumugam, D. Chinnaalagu, S. Samayanan, and S. Mahalingam (2021). Adv. Powder Technol. 32, 2213. https://doi.org/10.1016/J.APT.2021.04.030.
X. Zhao, W. Zhang, Y. He, L. Wang, W. Li, L. Yang, et al. (2021). Chemosphere 263, 127943. https://doi.org/10.1016/J.CHEMOSPHERE.2020.127943.
S. Setua, D. Menon, A. Asok, S. Nair, and M. Koyakutty (2010). Biomaterials 31, 714. https://doi.org/10.1016/J.BIOMATERIALS.2009.09.090.
S. Xing, L. Wang, C. Jiang, H. Liu, W. Zhu, and V. Ji (2020). Vacuum 181, 109665. https://doi.org/10.1016/J.VACUUM.2020.109665.
F. Huang, H. Wang, B. Yang, T. Liao, and Z. Wang (2019). Mater. Lett. 242, 119. https://doi.org/10.1016/J.MATLET.2019.01.120.
D. Marković, J. Vasiljević, J. Ašanin, T. Ilic-Tomic, B. Tomšič, B. Jokić, et al. (2020). J. Appl. Polym. Sci. 137, 49194. https://doi.org/10.1002/APP.49194.
W. V. da Costa, B. Pereira, S. M. C. da Montanha, E. Kimura, A. A. W. Hechenleitner, D. M. F. de Oliveira, et al. (2017). Mater. Chem. Phys. 201, 339. https://doi.org/10.1016/J.MATCHEMPHYS.2017.08.046
A. Regmi, J. Bhandari, S. Bhattarai, and S. K. Gautam (2019). J. Nepal Chem. Soc. 0304, 5. https://doi.org/10.3126/jncs.v40i0.27271.
Y. R. Rezende, J. P. Nogueira, and N. Narain (2018). Food Chem. 254, 281. https://doi.org/10.1016/j.foodchem.2018.02.026.
B. Guo, A. Harvey, S. H. Risbud, and I. M. Kennedy (2006). Philos. Mag. Lett. 86, 457. https://doi.org/10.1080/09500830600871194.
D. B. Pal, D. D. Giri, P. Singh, S. Pal, and P. K. Mishra (2017). Desalin. Water Treat 62, 355. https://doi.org/10.5004/DWT.2017.0201.
C. Sundaram, S. Kalpana, S. Rafi Ahamed, V. Sivaganesan, and E. Nandhakumar (2019). Mater. Res. Express 6, 125043. https://doi.org/10.1088/2053-1591/AB57D9.
H. Jo, Y.-I. Lee, M.-J. Suk, Y.-K. Jeong, and S.-T. Oh (2021). Arch. Metall. Mater. 66, 799. https://doi.org/10.24425/amm.2021.136383.
C. Guo, Z. Yu, C. Liu, X. Li, Q. Zhu, and Ward R. Mark (2020). Corros. Sci. 171, 108715. https://doi.org/10.1016/J.CORSCI.2020.108715.
D. Nunes, A. Pimentel, M. Matias, T. Freire, A. Araújo, F. Silva, et al. (2019). Nanomaterials 9, 234. https://doi.org/10.3390/NANO9020234.
K. Dulta, G. Koşarsoy Ağçeli, P. Chauhan, R. Jasrotia, P. K. Chauhan, and J. O. Ighalo (2022). Sustain. Environ. Res. 32, 1. https://doi.org/10.1186/S42834-021-00111-W/TABLES/4.
K. Phiwdang, S. Suphankij, W. Mekprasart, and W. Pecharapa (2013). Energy Procedia 34, 740. https://doi.org/10.1016/J.EGYPRO.2013.06.808.
N. Nasrollahi, S. Aber, V. Vatanpour, and N. M. Mahmoodi (2018). Mater. Chem. Phys. 222, 338. https://doi.org/10.1016/j.matchemphys.2018.10.032.
M. A. Bhosale, S. C. Karekar, and B. M. Bhanage (2016). ChemistrySelect 1, 6297. https://doi.org/10.1002/SLCT.201601484.
A. Khurana, P. Anchi, P. Allawadhi, V. Kumar, N. Sayed, G. Packirisamy, et al. (2019). Nanomedicine 18, 54. https://doi.org/10.1016/J.NANO.2019.02.018.
P. C. Nagajyothi, M. Pandurangan, M. Veerappan, D. H. Kim, T. V. M. Sreekanth, and J. Shim (2018). Mater. Lett. 216, 58. https://doi.org/10.1016/J.MATLET.2017.12.081.
I. Hamadneh, H. Alhayek, A. Al-Mobydeen, A. A. Jaber, R. Albuqain, S. Alsotari, et al. (2019). Egypt J. Chem. 62, 573. https://doi.org/10.21608/EJCHEM.2018.5281.1469.
S. K. Kannan and M. Sundrarajan (2015). Bull. Mater. Sci. 38, 945. https://doi.org/10.1007/S12034-015-0927-7/FIGURES/8.
A. S. Adeleye, S. Pokhrel, L. Mädler, and A. A. Keller (2018). Water Res. 132, 12. https://doi.org/10.1016/J.WATRES.2017.12.069.
V. M. Mani, S. Kalaivani, S. Sabarathinam, M. Vasuki, A. J. P. G. Soundari, M. P. Ayyappa Das, et al. (2021). Environ Res. 201, 111502. https://doi.org/10.1016/J.ENVRES.2021.111502.
V. T. Nguyen and K. S. Trinh (2020). J Chem. https://doi.org/10.1155/2020/9541934.
M. J. Woźniak-Budych, B. Maciejewska, Ł Przysiecka, D. Wieczorek, K. Staszak, J. Jenczyk, et al. (2020). J. Mol. Liq. 319, 114086. https://doi.org/10.1016/J.MOLLIQ.2020.114086.
S. M. Hasheminya, R. Rezaei Mokarram, B. Ghanbarzadeh, H. Hamishekar, and H. S. Kafil (2018). Food Packag. Shelf Life 17, 196. https://doi.org/10.1016/J.FPSL.2018.07.003.
J. Ren, W. Wang, S. Sun, L. Zhang, L. Wang, and J. Chang (2011). Ind. Eng. Chem. Res. 50, 10366. https://doi.org/10.1021/IE2005466/SUPPL_FILE/IE2005466_SI_001.PDF.
C. Angelé-Martínez, K. V. T. Nguyen, F. S. Ameer, J. N. Anker, and J. L. Brumaghim (2017). Nanotoxicology 11, 278. https://doi.org/10.1080/17435390.2017.1293750.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sector.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no declarations of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Długosz, O., Lis, K., Matyjasik, W. et al. Cu2O Nanoparticles Deposited on Y2O3 and CuO: Synthesis and Antimicrobial Properties. J Clust Sci 34, 2153–2165 (2023). https://doi.org/10.1007/s10876-022-02375-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-022-02375-7