Abstract
Barium titanate (BaTiO3) nanoparticles (BTNPs) have been considered as emerging materials in biomedical sector through last decades due to the excellent physicochemical properties such as dielectric and piezoelectric structures, biocompatibility, and nonlinear optical characteristics. In this study, BTNPs were synthesized via the co-precipitation method using barium carbonate and titanium dioxide by stirring for 5 h. Then, it was annealed at 850 °C for 5 h with five different concentrations: 0.2, 0.4, 0.6, 0.8, and 1 g/mL. The structural, morphological, and optical analyses were demonstrated by different characterization techniques such as X-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive X-ray (EDX), thermogravimetric analysis (TGA), Raman, and UV–visible spectroscopy. The perovskite phase of BTNPs, an intense peak at 31.6°, was observed at the lowest concentration (0.2 g/mL), and the average crystalline size was 1.42 nm based on XRD pattern. The results have been justified by SEM and EDX. TGA demonstrated the adequate thermal stability of this material. EDX analysis confirmed the composition of Ti, Ba, and O elements. Raman peaks at 305 cm−1 and 517 cm−1 confirmed the formation of BaTiO3. UV–visible spectra presented that its’ absorbance edge shifted into visible range at 404 nm. Application of BTNPs on breast cancer cell line (MCF-7) presented significant dispersion effect at 0.2, 0.4 and 0.6 g/mL of BaTiO3. A strong toxicity rate of BaTiO3 has been observed against the MCF-7 cell line. Maximum % of cell viability loss, \(\cong\) 57% was recorded at 200 µg/mL of BTNPs, and minimum % of cell viability loss was observed as 19% at 50 µg/mL of BTNPs. The results presented that a higher concentration of BTPNs dosage was more effective in inhibition of breast cancer cells. Therefore, BTNPs can be recommended as a promising nanomaterial for anti-cancer drug discovery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Throughout last several decades, cancer became one of the top deadly diseases worldwide [1]. Report says an average of 1.8 M cancer cases are investigated only in the United States every year [2]. The most popular remedies for cancer treatment are chemotherapy, radiotherapy, and surgery. However, there are several limitations in chemotherapy and radiotherapy, such as invasiveness [3,4,5]. To overcome this limitation, nanotechnology have been investigated comprehensively nowadays. Nanotechnology plays a vital role, especially in biomedical sector, such as targeted drug delivery systems. Recently, nanotechnology is practiced for various aspects of life science such as medicine, physics, chemistry, and molecular biology. Nanotechnology contains lots of capability to overcome the limitations of traditional cancer drugs and can open a new window for cancer drug discovery remove the disadvantages of traditionally used drug delivery [3,4,5].
BaTiO3 is a typical perovskite material having exclusive physical and chemical properties, which can be administered by its morphology and size. Therefore, nanoparticles are required to be highly purified for practical applications [6]. Barium titanate is insoluble in water and bases but soluble in acids like sulfuric acid and hydrochloric acid. Its’ bandgap is 3.2 eV at room temperature; however, it may increase upto ~ 3.5 eV while the element length is lower from approximately 15–7 nm [7]. BaTiO3 is a ferroelectric perovskite oxide [8], and it is primarily used in multilayer ceramic capacitors because of its’ great value of dielectric constant and lower loss properties [9]. BaTiO3 is the most studied material in the perovskite family and is extensively used to prepare multilayer ceramics capacitors [10]. Most inorganic nanomaterials are introduced in nanomedicine, but BaTiO3 is still unfamiliar. However, it can be good candidate for nanomedicine application due to its’ good biocompatibility, piezoelectric properties, and nonlinear features. Besides, BaTiO3 has therapeutic applications such as cancer therapy and drug delivery applications. Due to the inspiring conclusions of biocompatibility, it has been initiated into nanomedicine [11]. In 2010, therapeutic applications of BaTiO3 were implemented for first time. It has ability to absorb and deliver doxorubicin to SH-SY5Y. For this purpose, BaTiO3 was non-covalent with the drug [11].
The healing ability of barium titanate nanoparticles branches with the excellent piezoelectric coefficients of BaTiO3 has a tetragonal crystal-like phase [8]. Nanoparticle piezoelectricity is appealing for correspondence in tissue manufacturing due to the piezoelectric influence encouraging bone development or dreadful tissue re-establishes [12]. BaTiO3 nanoparticles had a robust potential to be bid as a multitasking antitumor agent [13]. A ceramic ingredient formed entirely on perovskite-like oxides has dragged attention for biomedical sector because it has both electrical and digital device forming applications. It is categorized as employing a high dielectric constant. BaTiO3 could be one of the highly deliberate elements of the perovskite group of families in near future. BaTiO3 is utilized commercially to produce multilayer thermoelectric applications with an effective temperature coefficient of resistivity [14, 15]. In addition, it has an excellent variety of inorganic nanoparticles examined in nanomedicine. BaTiO3 nanoparticles are still confined into laboratory-based research scale while it has high capabilities of precise biocompatibility, piezoelectric residences, and nonlinear optical characteristics. BTNPs might be used for various applications of nanomedicine, along with nonlinear imaging aims, drug carrier, tissue manufacturing, and bio stimulation [16,17,18,19,20]. Some recent experimental studies on BTNPs have been demonstrated on cancer therapy, molecular imaging, and non-invasive neurostimulation [21]. BaTiO3 nanoparticles have been synthesized using different methods such as sol–gel, co-precipitation, hydrothermal, and many more. Barium chloride and titanium tetrachloride are used in the sol–gel method, while barium carbonate, barium nitrate, and titanium dioxide are used in the precipitation method. In this study, Barium hydroxide and titanium hydroxide were implemented through the hydrothermal method which is expected to contribute a significant role in biomedical applications.
The main objective of this study is to investigate anti-cancer activity of BaTiO3 Previous experimental studies have presented that BaTiO3 was used primarily in ceramics. However, recently studies on barium titanate have been practiced for biomedical sector due to its’ excellent biocompatibility. In previous studies, BaTiO3 has been implemented on the different cancer cell lines such as BT-549, and MDA-MB-231 and presented excellent anti-cancer activity by BaTiO3. Therefore, in this current study, this nanocomposite has been targeted to inhibit the most common cancer cell line, MCF-7 to inhibit breast cancer [22].
Materials and Methods
Materials
Barium Carbonate (BaCO3), titanium dioxide (TiO2), ammonia (NH3), pure ethanol, fetal bovine serum (FEB), streptomycin, penicillin, fungizone, l-glutamate, neutral red assay (NRA), calcium chloride (CaCl2) and formaldehyde were purchased from Sigma Aldrich, USA. All chemicals were of analytical grade, and purity was 99.9%. Samples were prepared via the co-precipitation method [23].
Sample Preparation
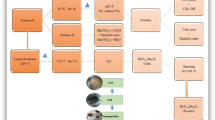
BTNPs were prepared via the co-precipitation method using BaCO3, TiO2, NH3, and deionized water. 1.97 g of BaCO3 was dissolved in 50 ml deionized water and stirred for 5-10 min to make it homogeneous. 0.79 g TiO2 was dissolved in 50 ml deionized water and stirred for 5–10 min to make it homogeneous. Then, both solutions were mixed in a beaker and continuously stirred for 6 h. The pH of the solution was measured as 5. Several drops of NH3 were added to maintain the pH of the solution up to 9. After 6 h of reaction, the solution was left at room temperature for cooling down. Precipitates were filtered with a Brazilian sheet and washed consecutively five times with deionized water and three times with ethanol. Washed precipitated were dried in the oven at 80 °C for 90mints. Then precipitates were ground for two h and annealed in the muffle furnace at 850 °C for 5 h. Figure 1 presented the schematic diagram of the different preparation stages of BTNPs.
Sample Characterizations
The synthesized BTNPs were characterized through different techniques. The structural configurations were studied by XRD (Bruker D8 Advanced, USA). The crystalline size, dislocation density, and micro-strain of the nanoparticles have been obtained by XRD analysis. The Scherrer formula is used to calculate the crystalline size, followed by Eq. 1.
where D is crystalline Size, k is the Sherrer constant (0.9), λ is the X-ray wavelength (0.154 nm), β is the full-wave half maxima, and \(\theta\) is Bragg’s angle. The dislocation density and micro-strain have been calculated by Eqs. 2 and 3, respectively.
Morphological structures were analyzed by SEM (EmCraft 100 CUBE series, Korea). The vibrational modes and rotational modes of BTNPs were studied by Raman spectroscopy (MN STEX-PRI 100, United Kingdom), and optical properties of nanoparticles were observed in UV–visible spectroscopy (PerkinElmer, Germany). The optical property, bandgap (Eg), was calculated by Eq. 4.
where A is constant, α is optical absorbance, ν is frequency, h is Planck’s constant (6.63 × 10–34), and n represents the nature of band transition (n = 1/2 and n = 3/2, shows the direct and indirect bandgap).
The thermal stability and the detailed elemental analyses were demonstrated by thermogravimetric analysis (TGA) (PerkinElmer, Germany) and Energy Dispersive X-ray (EDX) analysis (EmCraft 100 CUBE series, Korea), respectively.
Cell Culturing
MCF-7 cell line cultured in minimum essential medium (MEM) which contain 10% of fetal bovine serum (FEB), 100 µg/mL streptomycin, 100 µg/mL penicillin, 100 µg/mL fungizone, and 2 mM l-glutamate. The cell line was incubated in humidified 5% CO2 at 37 °C. When 75% of cell confluence was attained, further steps were performed [24].
Quantification of Cellular Uptake and Incubation Time
The breast cancer cell line, MCF-7, was incubated in 96 well plates for 24 h. Laser has been scattered on the solution of BaTiO3 with concentration (0.2–1 g/mL). All steps were demonstrated at room temperature. The cytotoxic and phototoxic effect of BaTiO3 on the MCF-7 cell line has been analyzed using a microplate reader [25].
Cell Viability
The MCF-7 cell line was incubated with various concentrations of BaTiO3 under dark conditions and irradiated by laser light, and then cell viability was then studied by neutral red assay (NRA). Then the medium containing BaTiO3 was replaced with a fresh medium containing NRA (50 µg/ml) and incubated for 4 h. Now, the medium was detached and washed cultured with the mixture of 10% calcium chloride (CaCl2) and 40% formaldehyde (v/v = 1:4). 50% ethanol and 1% acetic acid were added to remove red assay and impurities. The plate was shaken vigorously for 5 min and then left at room temperature for 15 min. The cells were quantified at 450 nm and compared with live-cell numbers. In parallel, a control microplate without BaTiO3 was prepared and exposed to NRA [26]. Cellular viability with various concentrations of BaTiO3 was evaluated and calculated by Eq. 5.
where \({P}_{treated}\) is the mean absorbance of BaTiO3 nanoparticles is, \({P}_{empty}\) is mean absorbance of empty wells, \({P}_{control}\) is the absorbance of control cells.
The optical absorption coefficient can be calculated by using Eq. 6:
where, d is the thickness of nanostructures, and T is transmittance.
Statistical Analysis
The results outcome, especially in terms of MTT assay analysis measured as mean ± standard deviation in triplicate time. Origin 9.1, 64-bit version software employed to assess data students t-test and value of p ≤ 0.05 measure statistical significance and by applying statistical by rows for determination of error possibility [25].
Results and Discussions
Structural Analysis
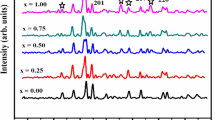
Figure 2 showed the XRD data of BaTiO3 nanoparticles annealed at 850 °C and prepared by the co-precipitation technique. In this experimental study, five samples were prepared with different concentrations. The concentration was increased from 0.2 g/mL to 1 g/mL. For the four concentrations with 0.4 to 1 g/mL, different peaks have appeared at 2θ = 27°, 36°, 39°, 41°, 45°, 54°, and 56° with miller indices are (130), (121), (111), (301), (002), (600) and (112) respectively [27]. Seven peaks were obtained, three significant peaks and four minor peaks. The most intense peaks were obtained at 27°, 36°, 41° and 54°, which belong to the non-perovskite phase of barium titanate (BaTiO3). Other phases were obtained at 2θ = 39°, 45°, and 56° belonging to the perovskite phase of BaTiO3. With the lowest concentration of 0.2 g/mL, the non-perovskite phase disappeared entirely, and the perovskite phase of BaTiO3 at 2θ = 22.1°, 31.6°, 39°, 45° 56° have been observed. The most intense peak showed strong BaTiO3 content with miler indices 101, and other miler indices are (001), (101), (111), (002), and (112) [28].
Figure 3 presented the relationship between FWHM and crystalline size of BaTiO3. Table 1 presented the different structural parameters of BTNPs. As the value of FWHM increases, crystalline size is decreased.
Raman Spectroscopy Analysis
Figure 4 shows the Raman spectra of BaTiO3 nanoparticles peak at 305 cm−1 along with another minor peak. XRD also confirmed the results [29]. There are two major peaks in 1st four samples (0.2 g/mL, 0.4 g/mL, 0.6 g/mL and 0.8 g/mL). The peak at 450 cm−1 belongs to BaCO3, and the peak at 610 cm−1 confirms the presence of BaTiO3 nanoparticles [30]. For the sample containing 1 g/mL concentration, all peaks disappeared while two new peaks were generated at 305 cm−1 and 517 cm−1, which belong to BaTiO3, verified by XRD data.
Scanning electron microscopy (SEM) analysis
Figure 5 presented the SEM images of BTNPs at different concentrations. Figure 5a–e presented the SEM images of BTNPs at 0.2 g/mL, 0.4 g/mL, 0.6 g/mL, 0.8 g/mL and 1 g/mL, respectively. In SEM images, random distribution of grain size was observed, and they are agglomerated. These SEM images showed crystalline structure at the surface of BTNPs with higher concentrations, especially with 0.8 g/mL and 1 g/mL. When the concentration decreased, structure crystallinity also decreased. XRD results also verified the crystal structure of BaTiO3 at higher concentrations. Therefore, 0.2 g/mL, 0.4 g/mL and 0.6 g/mL can be considered as higher potential doses for cancer cell inhibition.
TGA analysis
TGA analysis shows the measurement of change in weight of the given samples concerned with increment, cooled, or said at a constant temperature [31]. The TGA/DC results show the inverse relationship. When the temperature is less, the value of weight % is maximum, but as the value of temperature increases, and the value of weight % decreases. On the other hand, it is translucent that as soon as temperature increased above 200 ℃, the heat flow started to increase. In Fig. 6, during temperature ranges 32–600 °C, represents the removal of impurities (other precursors, impurities, etc.) took place. But, from 600 to 800 ℃, the complete weight loss of Ba took place while Ti was still present entirely since the melting point of Ti is higher than Ba, which is 1668℃.
EDX Analysis
EDX analysis presented in Fig. 7 depicts the elemental composition analysis.; In Fig. 7, relevant elements e.g., Au is used as base, and its’ presence is 2.19% in M series base materials during this characterization. As analysed in EDX presented in Table 2, O, K and Ti were 47.66%, 2.07% and 45.68% (wt), respectively in K series. Au was 2.4% (wt) in M series. The Au signal peaks corresponds to the sample holder while analyzing Barium titanate (BaTiO3).
UV–Vis Spectroscopy Analysis
Figure 8 represents the optical absorbance of BaTiO3 in the range of 200–1100 nm. It was observed that variation in peak, such as absorbance, varies with the wavelength variation. Figure 9 shows the bandgap of BaTiO3, plotted between energy verse (αhν)2, and the bandgap of BaTiO3 is 3.76 eV [26]. In the current study, the value of n is 2 because it shows the direct transition of material. Bandgap energy of the sample can be predicated zero when the value of αhν)2 is zero [32]. It is clear in the visible region; Barium Titanate NPs are very clear. The optical absorption coefficient depends upon the energy of the photon, which is used to help analyze band structure and type of transition electron [33].
Cell Growth Analysis
MCF-7 cell lines were cultured and maintained under certain suitable conditions of the ethical and animal committee of WHO recommendation. BaTiO3 NPs were applied on MCF-7 cell line with different concentrations (0.2 g/mL, 0.4 g/mL and 0.6 g/mL). The growth of cells was examined under dark conditions and LED light. Figure 10 showed the cell growth under dark conditions. In the first four days, the growth of cells increased. After that, the cell growth was almost steady. The maximum growth factor was obtained 63. The maximum concentration presented maximum growth.
Figure 11 showed the cell growth under the LED light. In the first four days, the cell growth increased. After that, the cell growth remained almost steady. The maximum growth factor is 40. The maximum concentration obtained maximum growth.
Cell Viability Analysis
The percentage (%) of cell viability loss of BaTiO3 nanoparticles was evaluated at the different concentrations (50 µg/mL, 100 µg/mL, 150 µg/mL, and 200 µg/mL). It showed the effect of BaTiO3 nanoparticles with different concentrations (0.2 g/ml, 0.4 g/ml and 0.6 g/ml) on MCF-7 cancer cell line. Figure 12 presented the cell viability rate by the different concentrations of BaTiO3 nanoparticles. The maximum % cell viability loss (\(\cong\) 57%) was recorded by a microplate reader using 490 nm and 510 nm filter at 200 µg/mL when exposed to MCF-7 cells. The minimum % cell viability loss was observed at 19% at 50 µg/mL dispersion of BaTiO3 NPs. This outcome shows that novel nanoparticles are feasible in cancer activity.
The experimental outcome of this study presented that the cell viability value decreases as the concentration increases from 25 to 200 µg/mL. An earlier experimental study by Maqusood Ahamed et al. presented the effective inhibition rate of BaTiO3 nanoparticles on human lung cancer cells. This study also observed the effect of BaTiO3 nanoparticles for different time intervals (24 h, 48 h, and 72 h). As the time duration increase, % loss is also decreased [34]. Another similar experimental study for various cell lines was conducted by Alam et al. [35,36,37,38,39,40,41,42,43,44]. Many researchers have focused on the anti-cancer and antitumor activity of BaTiO3. Cytotoxicity of BaTiO3 is time-dependent and dose manner. This nanoparticle also targeted mitochondrial membrane for apoptotic effect by liberating caspase-3 and 9-enzyme and inhibited lung cancer cells in earlier study [19]. In another study, BaTiO3 acted as a ROS scavenger via oxidative stress and reactive oxygen species (Ros Production). This activity manifests the ability of cancer cell inhibition potential via loss of mitochondrial membrane [13].
Conclusions
This study presented BaTiO3 nanoparticles as an effective nanomaterial for human breast cancer cell inhibition. Different characterizations of BaTiO3 nanoparticles presented the material with high stability. XRD results confirmed that the four samples with a 0.4-1 g/mL concentration were obtained the non-perovskite BaTiO3. While the concentration reached the lowest concentration of 0.2 g/mL, the pure perovskite phase of BaTiO3 was obtained. Raman spectroscopy and SEM results justified the XRD results. The optical properties of BaTiO3 analyzed by UV–visible spectroscopy indicated its bandgap, 3.76 eV. EDX measurements confirmed the composition of Ti, Ba and O element. The growth factor of MCF-7 cells was analyzed during incorporation of cancer cell lines withBaTiO3 NPs. The results demonstrated that only 0.2 g/mL, 0.4 g/ml, and 0.6 g/mL of BaTiO3 dispersion have significant effects. Anti-cancer activity of BaTiO3 nanoparticles on the MCF-7 cell line demonstrated high cell toxicity. The maximum cell inhibition rate was obtained with maximum BaTiO3 nanoparticles dosage. Therefore, BaTiO3 nanoparticles can be recommended as potential nanomaterials to be explored in commercial anti-cancer drug production in the future. However, this study presented some limitations of BaTiO3 such as BaTiO3 might be brittle ceramic and self-discharge, may cause high leakage due to thermal stress.
References
H. Pain, H. Hamavid, M. Moradi-Lakeh, M. F. MacIntyre, C. Allen, G. Hansen, R. Lozano, T. Vos, M. Forouzanfar, A. Lopez, C. Murray, and M. Naghavi (2015). The Global burden of cancer 2013. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2015.0735.
C. Y. Zhao, R. Cheng, Z. Yang, and Z. M. Tian (2018). Nanotechnology for cancer therapy based on chemotherapy. Molecules 23, 826.
K. H. Bae, H. J. Chung, and T. G. Park (2011). Nanomaterials for cancer therapy and imaging. Mol. Cells 31, 295–302.
S. Yasri and V. Wiwanitkit (2018). Applied biointerface technology for medical diagnosis: a summary. Biointerface Res. Appl. Chem. 8, 3490–3492.
A. Narayana (2014). Applications of nanotechnology in cancer: a literature review of imaging and treatment. J. Nucl. Med. Radiat. Ther. 5.
S. Adireddy, C. Lin, B. Cao, W. Zhou, and G. Caruntu (2010). Solution-based growth of monodisperse cube-like BaTiO3 colloidal nanocrystals. Chem. Mater. 22 (6), 1946–1948.
G. Manish, S. Vimukta, Targeted drug delivery system: a review. Res. J. Chem. Sci., 1(2), (2011).
G. Ciofani, S. Danti, D. D’Alessandro, S. Moscato, M. Petrini, and A. Menciassi (2010). Barium titanate nanoparticles: highly cytocompatible dispersions in glycol-chitosan and doxorubicin complexes for cancer therapy. Nanoscale Res. Lett. 5, 1093–1101.
W. S. Clabaugh, R. Swiggard, and R. Gilchrist (1956). Preparation of barium titanyl oxalate tetrahydrate for conversion to barium titanate of high purity. J. Res. Nat. Bur. Stds. 56, 289–291.
Y. Sakabe, K. Minai, and K. Wakino (1981). High-dielectric constant ceramics for base metal monolithic capacitors. Jpn. J. Appl. Phys. Suppl. 20–4, 147–150.
M. M. Vijatović, J. D. Bobić, and B. D. Stojanović (2008). History and challenges of barium titanate: II. Sci. Sinter. 40, 235–244.
G. G. Genchi, A. Marino, A. Rocca, V. Mattoli, and G. Ciofani (2016). Barium titanate nanoparticles: promising multitasking vectors in nanomedicine. Nanotechnology 27, 232001.
M. Giladi, et al. (2017). Tumor treating felds (TTFields) delay DNA damage repair following radiation treatment of glioma cells. Radiat Oncol 12, 206.
J. Wood, Doxorubicin, in M. Allwood, A. Stanley, and P. Wright (eds.), The Cytotoxic Handbook, 4th ed. (Radcliffe Medical Press Ltd, Oxon, 2002), pp. 322–329.
T. Jaglin (2007). Composite materials with viscoelastic stiffness greater than diamond. Science 315 (5812), 620–622.
F. Jianqing, Y. Huipin, Z. Xingdong (1997). Promotion of osteogenesis by a piezoelectric biological ceramic. Biomaterials 18, 1531−1534
A. Marino, et al. (2019). Piezoelectric barium titanate nanostimulators for the treatment of glioblastoma multiforme. J Colloid Interface Sci 538, 449–461.
R. S. Nacer, B. A. K. Silva, R. R. da Poppi, D. K. M. Silva, V. S. Cardoso, J. R. J. Delben, and A. A. S. T. Delben (2015). Biocompatibility and osteogenesis of the castor bean polymer doped with silica (SiO2) or barium titanate (BaTiO3) Nanoparticles. Acta Cir. Bras. 30, 255–263.
Tomas Jordan, Mikaela A. O’Brien, Catalina-Paula. Spatarelu, and Geoffrey P. Luke (2020). Antibody-conjugated barium titanate nanoparticles for cell-specific targeting. ACS Appl. Nano Mater. 3, 2636–2646.
M. M. Amiji (2006). Nanomedicine 2, 299.
Y. N. Yoon, D. S. Lee, H. J. Park, and J. S. Kim (2020). Barium titanate nanoparticles sensitise treatment-resistant breast cancer cells to the antitumor action of tumour-treating fields. Sci. Rep. 10 (1), 1–9.
M. Atif, S. Firdous, R. Mahmood, M. Fakhar-e-Alam, S. S. Z. Zaidi, R. Suleman, et al. (2011). Cytotoxic and photocytotoxic effect of photofrin® on human laryngeal carcinoma (Hep2c) cell line. Laser Phys. 21 (7), 1235–1242.
S. V. Ganachari, V. B. Patil, N. R. Banapurmath, M. E. M. Soudagar, K. Shahapurkar, A. Elfasakhany, et al. (2021). The investigation of mixed ferrofluids containing iron oxide nanoparticles and microspheres. Adv. Mater. Sci. Eng.
S. Iqbal, M. Fakhar-e-Alam, F. Akbar, M. Shafiq, M. Atif, N. Amin, et al. (2019). Application of silver oxide nanoparticles for the treatment of cancer. J. Mol. Struct. 1189, 203–209.
Y. Feng, F. Li, J. Yan, X. Guo, F. Wang, H. Shi, et al. (2021). Pan-cancer analysis and experiments with cell lines reveal that the slightly elevated expression of DLGAP5 is involved in clear cell renal cell carcinoma progression. Life Sci. 287, 120056.
A. Alshoaibi, O. Saber, and F. Ahmed (2021). Enhancement of optical activity and properties of barium titanium oxides to be active in sunlight through using hollandite phase instead of perovskite phase. Crystals 11 (5), 550.
A. A. Shah, A. Khan, S. Dwivedi, J. Musarrat, and A. Azam (2018). Antibacterial and antibiofilm activity of barium titanate nanoparticles. Mater. Lett. 229, 130–133.
M. Singh, B. C. Yadav, A. Ranjan, M. Kaur, and S. K. Gupta (2017). Synthesis and characterization of perovskite barium titanate thin film and its application as LPG sensor. Sens. Actuators B 241, 1170–1178.
S. Kumar, G. L. Messing, and W. B. White (1993). Metal organic resin derived barium titanate: I, formation of barium titanium oxycarbonate intermediate. J. Am. Ceram. Soc. 76 (3), 617–624.
S. Sikarwar, B. C. Yadav, S. Singh, G. I. Dzhardimalieva, S. I. Pomogailo, N. D. Golubeva, A. D. Pomogailo (2016). Sens. Actuators B 232, 283–291.
M. Ahamed, M. J. Akhtar, M. A. Khan, H. A. Alhadlaq, and A. Alshamsan (2020). Barium titanate (BaTiO3) nanoparticles exert cytotoxicity through oxidative stress in human lung carcinoma (A549) cells. Nanomaterials 10 (11), 2309.
S. Sagadevan and J. Podder (2015). Investigation of structural, SEM, TEM and dielectric properties of BaTiO3 nanoparticles.
J. S. Yaradoddi, N. R. Banapurmath, S. V. Ganachari, M. E. M. Soudagar, N. M. Mubarak, S. Hallad, et al. (2020). Biodegradable carboxymethyl cellulose based material for sustainable packaging application. Sci. Rep. 10 (1), 1–13.
S. Abbas, S. Nasir, M. Fakhar-e-Alam and M. Saadullah (2019). Toxicity of different groups of insecticides and determination of resistance in Aedes aegypti from different habitats. Pak. J. Agric. Sci. 56(1).
M. W. Akram, M. F. Alam, H. N. Ji, A. Mahmood, T. Munir, M. Z. Iqbal, et al. (2019). Chitosan blend iron oxide nanostructure-based biosensor for healthy & malignant tissue glucose/urea detection, in IOP Conference Series: Materials Science and Engineering, vol. 474, no. 1 (IOP Publishing, Bristol, 2019), p. 012060.
M. W. Akram, M. Fakhar-e-Alam, A. R. Butt, T. Munir, A. Ali, K. S. Alimgeer, et al. (2018). Magnesium oxide in nanodimension: model for MRI and multimodal therapy. J. Nanomater.
M. W. Akram, F. Raziq, M. Fakhar-e-Alam, M. H. Aziz, K. S. Alimgeer, M. Atif, et al. (2019). Tailoring of Au-TiO2 nanoparticles conjugated with doxorubicin for their synergistic response and photodynamic therapy applications. J. Photochem. Photobiol. A 384, 112040.
M. Atif, S. Iqbal, M. Fakhar-e-Alam, Q. Mansoor, K. S. Alimgeer, A. Fatehmulla, et al. (2021). Manganese-doped cerium oxide nanocomposite as a therapeutic agent for MCF-7 adenocarcinoma cell line. Saudi J. Biol. Sci. 28 (2), 1233–1238.
M. Atif, S. Iqbal, M. Ismail, Q. Mansoor, L. Mughal, M. H. Aziz, et al. (2019). Manganese-doped cerium oxide nanocomposite induced photodynamic therapy in MCF-7 cancer cells and antibacterial activity. BioMed Res. Int.
M. H. Bilal, R. Mehmood, M. Fakhar-e-Alam, T. Munir, M. Saadullah, A. Mahmood, et al. (2021). Biocompatible graphene oxide (GO) nanobiosensor used for quantitative analysis of glucose. Dig J Nanomater Biostruct 16(1).
M. Fakhar-e-Alam, M. W. Akram, S. Iqbal, K. S. Alimgeer, M. Atif, K. Sultana, et al. (2017). Empirical modeling of physiochemical immune response of multilayer zinc oxide nanomaterials under UV exposure to melanoma and foreskin fibroblasts. Sci. Rep. 7 (1), 1–13.
M. Fakhar-e-Alam, M. Aseer, M. S. Rana, M. H. Aziz, M. Atif, N. Yaqub, and W. A. Farooq (2020). Spectroscopic features of PHOTOGEM® in human Rhabdomyosarcoma (RD) cellular model. J. King Saud Univ. Sci. 32 (7), 3131–3137.
M. Fakhar-e-Alam, M. Atif, K. S. Alimgeer, M. S. Rana, N. Yaqub, W. A. Farooq, and H. Ahmad (2020). Synergistic effect of TEMPO-coated TiO2 nanorods for PDT applications in MCF-7 cell line model. Saudi J. Biol. Sci. 27 (12), 3199–3207.
M. Fakhar-e-Alam, A. Mahmood, S. Nasir, M. Saadullah, M. W. Akram, and M. Willander, Gadolinium-doped iron nanostructures decorated with novel drugs for magnetic resonance imaging, photodynamic, and photothermal therapy applications, in Magnetic Nanoheterostructures (Springer, Cham, 2020), pp. 121–159.
Acknowledgements
The authors extend their appreciation to the Higher Education Commission Pakistan under NRPU Project Grant# 8056/Punjab/NRPU/R&D/HEC/2017.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
MFA: conceptualization, formal analysis, project administration, writing- review and editing, supervision; SS: methodology, formal analysis, writing- original draft; NH: supervision, writing- review and editing, proofreading; AS: formal analysis; IU: formal analysis; AS: formal analysis; JI: software.
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fakhar-e-Alam, M., Saddique, S., Hossain, N. et al. Synthesis, Characterization, and Application of BaTiO3 Nanoparticles for Anti-Cancer Activity. J Clust Sci 34, 1745–1755 (2023). https://doi.org/10.1007/s10876-022-02346-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-022-02346-y