Abstract
Chemical reactions of ball-mill activated [Nb6I11] with alcohol solutions of Mg alcoholates result in the formation of the three new hexanuclear Nb cluster compounds [Nb6I8(HOCH3)6][Nb6(OCH3)18]·2CH3OH (1), (PPh4)2[Nb6(OC2H5)12I6]·C2H5OH (2) and (PPN)2[Nb6(OC2H5)12I6] (3). They contain cluster anions, which have the edges of the Nb6 octahedra, i.e. the inner coordination sites, μ2-bridged by the O atoms of methanolato or ethanolato ligands. Thereby, they represent further characterized members of the so far very small group of hexanuclear cluster alcoholates. The terminal metal sites are bonded to further methanolato (1) or iodido ligands (2 and 3). Sterically demanding organic cations (PPh4+, PPN+, 2 and 3) or a cationic Nb6 cluster complex with terminal methanol ligands [Nb6I8(HOCH3)6]2+ in 1 compensate the negative charges of the cluster anions. Compound 1 is the first example of a M6 cluster ion pair of both the text-book known edge-bridged [M6X12] and face-bridged [M6X8] cluster units. The structures of the three title compounds were determined by means of single-crystal X-ray structure analysis.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Early transition metals in low oxidation states often form coordination compounds with polyhedral aggregates of metal atoms. These so-called clusters are characterized by strong metal–metal bonds [1, 2]. For the elements of the fourth and fifth transition metal group, a metal atom octahedron M6 often is the basic structural motif [3,4,5,6,7,8,9,10]. Depending on the bonding of the surrounding ligands, two different cluster types can be distinguished. The [M6X8] type denotes a metal atom octahedron, which has the octahedral faces coordinated by a total of eight ligands (inner ligand sphere). In the [M6X12] type, the inner ligands bridge the edges of the metal atom octahedron. In both types, the outer ligand sphere is spanned by six monodentate ligands at the corners of the metal atom octahedron, [M6Xi12Ya6] or [M6Xi8Ya6] (i: inner, a: outer, außen) [9]. Interstitial atoms can be incorporated into the metal atom octahedron. Examples are known for both cluster types. In addition to the structural diversity these cluster coordination compounds are also characterized by reversible redox activity. Compounds with 14, 15, or 16 cluster-based electrons (CBE’s) exist [11].
Cluster compounds are known, which consist either of isolated (discrete) units or units that are linked through bridging ligands [12, 13]. Solution-based chemical reactions allow for an exchange of ligands [14]. This enables complex compounds to be synthesized that are not accessible by means of conventional high-temperature synthesis. In most of these compounds, the six positions of the outer ligand sphere are primarily exchanged as a result of the ligand substitution. For M = Nb or Ta a large number of new compounds [M6X12L6] with L = nitriles [15,16,17], amines [13, 18, 19], alcohols [14, 17, 20, 21], water [22,23,24,25,26], etc. have been prepared and characterized. Due to the structural diversity and the many choices of ligands and therefore clusters with different properties, this interesting field of chemistry has been intensively researched for many years [3, 4, 7, 27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44].
The substitution of the inner ligands is challenging and has been achieved so far only for L = halide and alcoholate ligands. The cluster compound Na2[Mo6(OCH3)i8(OCH3)a6]·10CH3OH is the first example published. [45, 46] It was not until 2009 that the first Nb6 alcoholates, [K(HOCH3)4]2[Nb6(OCH3)18] and [Na([18]crown-6)(C2H5OH)2]2[Nb6(OC2H5)12(NCS)6] were synthesized [20], and in 2021 the Ta6 alcoholates, [Mg(HOCH3)6][Ta6(OCH3)18]·6CH3OH and An[Ta6(OC2H5)12I6] (A = PPh4, PPN; n = 1, 2) [47].
In the context of this publication, a compound is also presented, which consists of a cluster anion and a cluster cation. Such compounds are either heteroatomic such as [M6X12(HOC2H5)6]-
[Mo6Cl8Cl4X2]·nC2H5OH·m(C2H5)2O (M = Nb, Ta; X = Cl, Br) [48] or homoatomic [Ta6Cl12(PrCN)6][Ta6Cl12Cl6]·2PrCN [49] and [Nb6Cl12(RCN)6][Nb6Cl18] (R = C2H5, nPr, iPr) [50].
Experimental Section
Materials
If not otherwise stated starting materials were purchased from commercial suppliers and used as received. Methanol and ethanol (HPLC, Chemsolute, purity > 99.9%) were dried over molecular sieve (3 Å) for several days and after filtration refluxed under argon and carefully degassed.
Magnesium powder (Roth, ≥ 99.8%, particle size < 75 µm) and PPh4I (TCI, purity > 98%) were dried in a high vacuum (10–6 mbar) at 60 °C for several hours.
(PPN)I was synthesized according to a literature known method [47].
All preparative steps and materials handlings were carried out strictly under Schlenk conditions within closed, baked out apparatus or in a glove box. Some of the compounds are pyrophoric in air.
Single-Crystal X-ray Diffraction
Diffraction data of single-crystals were measured using either a Bruker-Nonius Apex Kappa II diffractometer with fine focus tube, monochromator, Oxford-Cryosystem Cooler and a CCD detector, or a Bruker D8 Quest diffractometer with micro-focus X-ray tube, Bruker-Kryoflex low temperature Cooler and an Apex-Smart CCD Pixel Array Detector [51]. Mo-Kα radiation, λ = 0.71073 Å, was used in both cases. Structure solutions and refinements were done with the aid of the Shelx-14 program package [52,53,54,55]. In the final structure refinements the hydrogen atoms were placed on positions assuming idealized geometry and refined using riding models. The handling of the programs was done with ShelXle [56]. Pictures of the compounds were prepared with the program DIAMOND, Version 4.6.3 [57].
In 2 some of the terminal bonded I atoms as well as one of the ethanolato ligands are disordered and refined on split positions.
NMR Spectroscopy
NMR data were collected with a Bruker AVANCE 300 and a Bruker AVANCE 500 device.
IR Spectroscopy
IR measurements were carried out as ATR experiments on a Bruker Alpha II spectrometer at RT and inert atmosphere.
Elemental Analyses
Elemental analyses (C, H, N) were performed on a Flash EA 112 Series device and handle under inert conditions.
Synthesis of the Starting Material [Nb6I11]
For the synthesis a slightly modified procedure of that published by A. Simon et al. in 1967 was used [58]. Inside a glove box, stoichiometric amounts of NbI5 (prepared from the elements, sublimated) and Nb powder (Chempur, ≥ 99.9%, < 65 µm) are finely ground and filled in 3 g portions into Niobium metal ampoules. These are welded shut in an arc furnace under protective gas. 3–4 metal ampoules are then placed in a 20 cm long and 2.5 cm wide quartz glass ampoule and these are sealed in a high vacuum (10–6 mbar). In the tube furnace, heating is carried out using a temperature program from RT to 800 °C within 2 days, holding at 800 °C for 2 days and cooling from 800 °C to RT within 1.5 days. After completion of the solid-state synthesis, the ampoules are opened under protective gas and a black-crystalline product is obtained. The completeness of the reaction and purity are checked using XRPD. To increase the solubility of [Nb6I11], the coarsely crystalline product is finely ground in a ball mill under protective gas. The powder obtained is pyrophoric in air.
Synthesis of the Cluster Compound [Nb6I8(HOCH3)6][Nb6(OCH3)18]·2CH3OH (1)
120 mg (61.43 µmol) of [Nb6I11] and 8.0 mL (0.198 mol) of methanol are placed in an 8.5 mL flask and heated at 60 °C for 12 h. After cooling, the black solution is filtered and 40 mg (1.646 mmol) of Mg powder are added. The reaction mixture is again heated at 60 °C for at least one week. The color of the solution changes from black to dark red. Large reddish-black single crystals form on the wall of the sample flask and some dark brown, amorphous precipitate. If the formation of single crystals does not occur in the reaction solution, the crystallization of the target compound can be made possible by further addition of smaller amounts of [Nb6I11]. The yield of crystalline product varies between 5 and 45% when the reaction is carried out identically. The product is ground in a mortar for further investigations and dried in a high vacuum (10–6 mbar) with slight warming for several hours. This removes the co-crystalline methanol from the compound.
Compound 1 is highly reactive and pyrophoric in air. Elemental analysis gave unreliable results due to the high reactivity of the cluster salt. Furthermore, 1 is insoluble in all common solvents. Only water containing DMSO dissolves 1 to a small extent. Water containing deuterated DMSO was used for the NMR measurements; thereby the alcoholato ligands of the cluster are separated from the cluster core and measured indirectly as alcohols.
1H-NMR ([D6]DMSO (H2O), 300 MHz, 300 K, ppm): δ = 3.17 (s, 72H, CH3OH), 4.08 (s, 24H, CH3OH).
13C{1H} NMR ([D6]DMSO (H2O), 300 MHz, 300 K, ppm): δ = 48.56 (s, CH3OH).
IR (300 K, ATR, cm−1): ν̃ = 425 (vs), 481 (s), 614 (m), 808 (m), 1013 (s), 1042 (s), 1434 (w), 2780 (w), 2811 (w), 2867 (w), 2914 (w).
Synthesis of the Compound (PPh4)2[Nb6(OCH3CH2)12I6]·C2H5OH (2)
1000 mg (511.94 µmol) [Nb6I11] and 60 mL (1.028 mol) ethanol are heated at 60 °C for 5 days under stirring (magnetic stirrer) in a 100 mL Schlenk flask. After cooling, the black solution is filtered. To a volume of 6 mL of this solution 20 mg (822.88 µmol) magnesium powder are added in a 8 mL sample flask and heated at 80 °C for one day. As a result of the reaction, the solution becomes intensely red–orange and amorphous sediment forms, which can be easily removed by centrifugation and subsequent filtration.
The red–orange starting solution is mixed with 100 mg (214.46 µmol) PPh4I and heated at 60 °C for at least two days. Large orange single crystals form on the wall of the sample flask. These are taken directly from the solution for the single crystal structure analysis. For further analyses the single crystals are washed several times with ethanol, ground in a mortar and dried in a high vacuum (10–6 mbar) for 6 h at 60 °C. This removes the co-crystalline ethanol.
Yield: 51 mg (85%).
Elemental analysis: M [C72H100I6Nb6O12P2] = 2538.362 g·mol−1: found C = 33.47% (calcd. 34.07%), H = 3.92% (3.97%).
1H-NMR ([D3]MeCN, 500 MHz, 300 K, ppm): δ = 1.19–1.40 (m, 36H, CH3CH2O), 4.49–5.22 (m, 24H, CH3CH2O), 7.62–7.78 (m, 32H, meta+orthoH-Ph), 7.87–7.96 (m, 8H, paraH-Ph).
13C{1H} NMR ([D3]MeCN, 500 MHz, 300 K, ppm): δ = 19.82 (s, CH3CH2O), 77.00 (s, CH3CH2O), 119.35 (s, ipsoC-Ph), 131.73 (m, metaC-Ph), 136.11 (m, orthoC-Ph), 136.80 (s, paraC-Ph).
IR (300 K, ATR, cm−1): ν̃ = 439 (m), 449 (s), 476 (s), 505 (vs), 524 (vs), 686 (s), 719 (s), 748 (m), 763 (m), 798 (m), 845 (m), 899 (s), 993 (m), 1039 (vs), 1090 (m), 1107 (m), 1185 (w), 1313 (w), 1350 (w), 1372 (m), 1437 (m), 1457 (w), 1482 (w), 1583 (w), 2855 (w), 2917 (w), 2962 (w), 3046 (w).
Synthesis of the Cluster Compound (PPN)2[Nb6(OCH3CH2)12I6] (3)
The synthesis of 3 is carried out analogously to 2. The red–orange starting solution is mixed with 100 mg (150.27 µmol) (PPN)I and heated at 60 °C for several days. Large orange single crystals form on the wall and bottom of the sample flask. These are taken directly from the solution for the single crystal structure analysis. For further analysis, the single crystals are washed several times with ethanol, ground in a mortar and dried in a high vacuum (10–6 mbar) for 6 h at 60 °C.
Yield: 42 mg (70%).
Elemental analysis: M [C96H120I6N2Nb6O12P4] = 2936.746 g·mol−1: found C = 39.08% (calcd. 39.26%), N = 0.95% (0.95%), H = 4.26% (4.12%).
1H-NMR ([D3]MeCN, 500 MHz, 300 K, ppm): δ = 1.19–1.37 (m, 36H, CH3CH2O), 4.49–5.24 (m, 24H, CH3CH2O), 7.42–7.52 (m, 24H, orthoH-Ph), 7.52–7.62 (m, 24H, metaH-Ph), 7.63–7.69 (m, 12H, paraH-Ph).
13C{1H} NMR ([D3]MeCN, 500 MHz, 300 K, ppm): δ = 19.72 (s, CH3CH2O), 76.43 (s, CH3CH2O), 128.63 (J: 109 Hz, ipsoC-Ph), 130.68–133.80 (m, metaC-Ph), 133.58–133.75 (m, orthoC-Ph), 135.00 (s, paraC-Ph).
IR (300 K, ATR, cm−1): ν̃ = 441 (m), 451 (s), 478 (s), 499 (vs), 532 (s), 548 (m), 688 (s), 721 (s), 746 (m), 796 (m), 901 (s), 996 (m), 1039 (s), 1090 (m), 1113 (m), 1060 (w), 1181 (w), 1243 (m), 1280 (w), 1352 (w), 1437 (m), 1457 (vw), 1482 (w), 1573 (vw), 1587 (w), 2855 (w), 2873 (w), 2912 (w), 2929 (w), 2962 (w), 3053 (w).
Results and Discussion
In this paper the synthesis and characterization of the three new hexanuclear niobium alcoholates with the formulas [Nb6I8(HOCH3)6][Nb6(OCH3)18]·2CH3OH (1), (PPh4)2[Nb6(OC2H5)12I6]·C2H5OH (2) and (PPN)2[Nb6(OC2H5)12I6] (3) (PPN: bis (triphenylphosphine) iminium, Ph: phenyl) are presented.
Synthesis
The first step of the formation of the three title cluster compounds is the excision of [Nb6I11] into anhydrous alcohols (for 1 methanol, for 2 and 3 ethanol). Ball milling of the precursor has been proven to be beneficial for the reactivity of the starting material [47]. In all three cases magnesium powder is added to the filtered solutions in large molar excess. This reacts quickly with the alcohol forming alcoholate anions. Under these strong basic conditions inner μ3 bridging iodido ligands are substituted and cluster units of the type [Nb6(OR)i12] with O-μ2 bridging ligands are formed. This means that the precursor of the [M6X8] cluster type is transferred into the [M6X12] type. Compound 1 crystallizes out of the reaction solution without further additives in moderate yield. It is a double cluster salt consisting of the [Nb6(OCH3)18]2– anion of the [M6X12] cluster type and [Nb6I8(HOCH3)6]2+ cations of the [M6X8] type. The cluster anion has all ligand positions occupied by methanolato ligands. Therefore, it is a metal cluster alcoholate. It contains 14 CBE’s. The cluster cation presumably forms already when the solid state precursor is dissolved in methanol. On adding salts of large organic cations to the Mg treated cluster precursor solution 2 and 3 crystallize. Both contain the same cluster anion, [Nb6(OC2H5)12I6]2–, which contains also 14 CBE’s.
Structures
The single crystal structures of the title compounds are determined by means of X-ray structure analysis. Details of the structure determinations are given in Table 1.
All three compounds consist of discrete cluster anions of the general formula [Nb6(OR)i12Ya6]2− (R = CH3 or C2H5, Ya = OCH3 or I) and the charge-compensated cations PPh4+, PPN+ or the cluster cation [Nb6Ii8(HOCH3)a6]2+. They represent further examples of electron-poor M6 cluster alcoholates, of which two compounds for M = Nb and four for M = Ta have been published before [20, 47]. The six outer coordination sites of the metal octahedra are occupied by methanolato ligands in 1 and iodido ligands in 2 and 3. With the exception of 3, the title compounds further contain co-crystalline solvent molecules. Compounds 1 and 2 crystallize in the triclinic crystal system with space group P \(\overline{1 }\) and compound 3 in the monoclinic crystal system, space group P21/c. All three title compounds contain octahedral Nb6 cluster anions, which have all inner coordination sites O-bonded to alcoholato ligands. The structure of the cluster cation/anion pair and the co-crystallized solvent molecule in 1 is shown in Fig. 1.
Structure of the cluster anion and the cluster cation, which are interconnected through hydrogen bonds via a co-crystallized methanol molecule in crystals of 1. Thermal displacement ellipsoids are drawn at the 50% probability level. The Nb6 octahedra are shown in a polyhedral representation and the hydrogen bonds as red dashed lines (Color figure online)
As mentioned above, compound 1 is unique, because it is the first example of a [M6X12] type cluster anion, which is charge compensated through a cluster unit of the face-bridged [M6X8] type. It has the formula [Nb6I8(HOCH3)6]2+ with the inner ligand sphere occupied by iodido and the outer by methanol ligands. In the cluster anion all ligands of the starting material [Nb6I11] are substituted by methanolato ligands (with the change of the face-bridged to the edge-bridged cluster type), whereas the cluster cation has kept the inner ligand of the starting material. This indicated that in solution different cluster species exist, which are linked through chemical equilibria. Selected interatomic distances within 1 are listed in Table 2.
The average Nb–Nb and Nb–O distances in the cluster anion of 1 compare well to those found in [K(HOCH3)4]2[Nb6(OCH3)18] (Nb–Nb: 2.873 Å, Nb–Oi: 2.081 Å, Nb–Oa: 2.148 Å) [20]. These distances are strongly correlated with the oxidation state of the Nb atoms and thereby the number of cluster based electrons, CBE’s. The number of CBE’s on the two cluster species in 1 depends strongly on the question of where hydrogen atom are located. The doubtless experimental assignment of H atoms to the OCH3 groups (neutral methanol or anionic methanolate) is difficult. As mentioned in the Experimental Sect. 1 is highly reactive towards air oxygen and water and also insoluble in most solvents. Furthermore, 1H and 13C NMR as well as IR measurements would not answer this question. Neutron diffraction would be the method of choice, but has not been applied because of the difficulties to access this method. From the structural data at hand and the comparison with the (so far very few, see above) known Nb6 alcoholates it is reasonable to assign the charge of − 2 to the edge-bridged cluster unit, which carries in this case 14 CBE’s. Thereby, the face bridged unit needs to compensate the charge what means + 2, which requires 20 CBE’s. In this case the cluster anion carries only anionic methanolate and the cation only neutral methanol ligands on the exo sites. Looking more closely at the structure of this compound, see Fig. 1, the two cluster ions are interconnected by the two co-crystalline methanol molecules through short and thereby strong hydrogen bonds. The donor–acceptor distances range from 2.471(3) to 2.581(4) Å with angles between 154° and 166°. Two of these are shown in Fig. 1 as red dashed lines. This can be interpreted as proton shift equilibrium according to Scheme 1.
Thereby the charges vary but the number of CBE’s is not changed on both units. We assume that in 1 charged units with strong ionic interactions are the dominant species, because of the low solubility. A formal oxidation, a removal of H+ without changing the charge is unreasonable, because face bridged Nb6 cluster units with less than 20 CBE’s have never been observed.
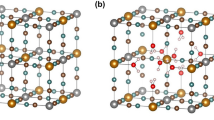
The packing of cluster units in crystals of 1 is shown in Fig. 2.
View of the packing of the discrete cluster units of 1 within and around the unit cell. The Nb6 octahedra are shown as polyhedra with those of the [Nb6(OCH3)18]2– anions in dark blue colors and those of the [Nb6I8(HOCH3)6]2+ cations in light blue (co-crystallized methanol molecules and H atoms are omitted for better visibility) (Color figure online)
This picture shows, that cluster cations and anions are packed in layers stacked along both the crystallographic b and c direction.
Compound 1 is to the best of our knowledge the first cluster pair compound, which contains ions of both the face-bridged [M6X8] as well as the edge-bridged [M6X12] cluster type.
The structures of the cluster anion and the respective organic cation pairs of 2 and 3 are shown in the Figs. 3 and 4.
View of the structure of the cluster anion and cation pair and the co-crystallized ethanol molecule in crystals of 2. Thermal displacement ellipsoids are drawn at the 50% probability level. The Nb6 octahedron is shown in a polyhedral representation. Of the disordered I atoms and ethanolato ligand only those with the larger occupational factors are shown
Both compounds contain the same cluster anion, [Nb6(OC2H5)12I6]2–, which has the inner octahedral sites coordinated by ethanolato and the terminal sites by iodido ligands. With two organic cations per cluster unit, PPh4+ in 2 and PPN+ in 3 the cluster charge is clearly defined as –2. Thereby, the CBE count is 14 for both compounds. This is nicely in line with the average Nb-Nb bond distances, which are 2.8555 Å in 2 and 2.8574 Å in 3, see Table 3. These distances agree well with those found in the cluster unit of [Na([18]crown-6)(HOC2H5)2]2[Nb6(OC2H5)12(NCS)6] (2.855 Å), which also contains 14 CBE’s [20]. Further selected atom distances within 2 and 3 are collected in Table 3. They all are found within the expected ranges.
Very similar compounds to 2 and 3 exist in the Ta analogues, (Ph4P)2[Ta6(OC2H5)12I6] and (PPN)2[Ta6(OC2H5)12I6] [47]. The last one is in fact isostructural to 3, whereas compound 2 differs from the Ta analogue by the existence of a co-crystallized ethanol molecule and thereby by a different lattice.
Whereas 1 contains acidic protons, which form hydrogen bonds (see structural discussion above), these are missing in 2 and 3. Therefore, in these cluster compounds no short hydrogen bonds are present. Also, π–π interactions are not found between aromatic rings of the counter cations.
Whereas for the group VB metals Nb and Ta octahedral M6 clusters are so far only known with alcoholato ligands occupying terminal or μ2 edge-bridging sites, for M = Mo examples are known with methanolato ligands on the μ3 face-bridging sites, e.g. in the [Mo6(OCH3)8(OCH3)6]2– anion [46]. The question arises, if such a coordination is also possible for M = Nb or Ta. Geometrical calculations show that in a hypothetical [Nb6(OCH3)8] unit assuming Nb–Nb and Nb–O distances as in the title compounds, the Nb–O–Nb angle is more close to ideal tetrahedral values than the Mo–O–Mo angle in the cluster unit mentioned above. Therefore, we propose that such compounds could be stable even though they have not been synthesized so far.
Conclusions
In summary, three new cluster compounds with hexanuclear, octahedral Nb6 anions, which are edge-bridged by alcoholato ligands were synthesized and structurally characterized. So far only two examples with such Nb6 alcoholate units have been known. The charge of the cluster anions in two of these compounds is balanced by sterically demanding organic cations. The third new cluster compound exhibits a double cluster salt, which has the charge of the cluster anion compensated by that of a cluster cation. This comprises a Nb6 cluster unit face-bridged by iodido ligands with terminal bonded methanol ligands ([M6X8] cluster type). Such an alcohol-alcoholate double cluster complex has not been previously described.
References
F. A. Cotton and T. E. Haas (1964). Inorg. Chem. 3, 10.
F. A. Cotton (1966). Q. Rev. Chem. Soc. 20, 389.
T. Hughbanks (1989). Prog. Solid State Chem. 19, 329.
C. Perrin (1997). J. Alloys Compd. 262–263, 10.
N. Prokopuk and D. F. Shriver (1998). Adv. Inorg. Chem. 46, 1.
A. Simon (1981). Angew. Chem. 20, 1.
A. Simon (1988). Angew. Chem. 100, 163.
H. S. Harned, C. Pauling, and R. B. Corey (1960). J. Am. Chem. Soc. 82, 4815.
H. Schäfer and H. G. von Schnering (1964). Angew. Chem. 76, 833.
P. A. Vaughan, J. H. Sturdivant, and L. Pauling (1950). J. Am. Chem. Soc. 72, 5477.
R. A. Mackay and R. F. Schneider (1967). Inorg. Chem. 6, 549.
C. Perrin and M. Sergent (1991). Eur. J. Solid State Inorg. Chem. 28, 933.
D. H. Weiß and M. Köckerling (2018). Chem. Eur. J. 24, 18613.
C. E. Runyan and T. Hughbanks (1994). J. Am. Chem. Soc. 116, 7909.
A. Flemming and M. Köckerling (2008). Z. Anorg. Allg. Chem. 634, 2309.
P. Planinić, V. Rastija, S. Širac, M. Vojnović, L. Frkanec, N. Brničević, and R. E. McCarley (2002). J. Cluster Sci. 13, 215.
E. Sperlich, J. König, D. H. Weiß, F. Schröder, and M. Köckerling (2019). Z. Anorg. Allg. Chem. 645, 233.
A. Flemming, J. König, and M. Köckerling (2013). Z. Anorg. Allg. Chem. 639, 2527.
F. Stollmaier and A. Simon (1985). Inorg. Chem. 24, 168.
A. Flemming and M. Köckerling (2009). Angew. Chem. Int. Ed. 48, 2605.
L. F. Piedra-Garza and M. Köckerling (2006). Inorg. Chem. 45, 8829.
N. Brničević, B. Kojic, and D. Plavsic (1981). Z. Anorg. Allg. Chem. 472, 200.
F. Schröder, M. Köckerling (2021). IUCrData 6, x210304.
M. V. Shamshurin, M. A. Mikhaylov, T. Sukhikh, E. Benassi, A. R. Tarkova, A. A. Prokhorikhin, E. I. Kretov, M. A. Shestopalov, P. A. Abramov, and M. N. Sokolov (2019). Inorg. Chem. 58, 9028.
B. Spreckelmeyer (1968). Z. Anorg. Allg. Chem. 355, 147.
M. Vojnović, N. Brničević, I. Bašic, P. Planinić, and G. Giester (2007). Crystallogr. Rep. 52, 239.
R. W. Berg (1992). Coord. Chem. Rev. 113, 1.
P. Braunstein, L. A. Oro, and P. R. Raithby, Metal Clusters in Chemistry (Wiley-VCH, Weinheim/Germany, 1999).
J. D. Corbett, in E. Parthé (ed.), Modern Perspectives in Inorganic Crystal Chemistry (Kluwer Academic Publ., Norwell, MA/USA, 1992).
J. D. Corbett (1996). Dalton Trans., 575.
J. D. Corbett (2000). Inorg. Chem. 39, 5178.
S. Dehnen, in D.M.P. Mingos (ed.), Clusters—Contemporary Insight in Structure and Bonding. Struct. Bonding (Springer, Berlin, 2017) vol. 174.
T. G. Gray (2003). Coord. Chem. Rev. 243, 213.
J.-F. Halet, Ligated Transition Metal Clusters in Solid-state Chemistry. Struct. Bonding (Springer, Berlin, 2019) vol. 180.
S. Kauzlarich, G. Meyer, and L. Chen (2011). Eur. J. Inorg. Chem. 2011, 3819.
R.B. King, in S.J. Lippard (ed.), Progress in Inorganic Chemistry (1972).
P. Lemoine, J.-F. Halet, and S. Cordier, in J.-F. Halet (ed.), Ligated Transition Metal Clusters in Solid-state Chemistry: The legacy of Marcel Sergent, (Springer, Cham, 2019).
Z. Lin and I. D. Williams (1996). Polyhedron 15, 3277.
E. L. Muetterties, T. N. Rhodin, E. Band, C. F. Brucker, and W. R. Pretzer (1979). Chem. Rev. 79, 91.
H. Schäfer and H.-G. von Schnering (1964). Angew. Chem. Int. Ed. 76, 833.
G. Schmid, Clusters and Colloids: From Theory to Application (VCH, Weinheim, 1994).
A. Simon, in G. Schmid (ed.), Clusters and Colloids (Verlag Chemie, Weinheim, 1994).
A. Simon (1995). Pure Appl. Chem. 67, 311.
A. Simon (2010). Phil. Trans. A-368, 1285.
M. H. Chisholm, J. A. Heppert, and J. C. Huffman (1984). Polyhedron 3, 475.
P. Nannelli and B. Block (1968). Inorg. Chem. 7, 2423.
F. Schröder and M. Köckerling (2021). Z. Anorg. Allg. Chem. 647, 1625.
I. Bašic, N. Brničević, U. Beck, A. Simon, and R. E. McCarley (1998). Z. Anorg. Allg. Chem. 624, 725.
N. Brničević, S. Širac, I. Bašic, Z. Zhang, R. E. McCarley, and A. G. Ilia (1999). Inorg. Chem. 38, 4159.
E. Sperlich and M. Köckerling (2020). Z. Naturforsch. B 75, 173.
Bruker, APEX3, SADABS and SAINT (Bruker AXS Inc., Madison, 2017).
G. M. Sheldrick (1990). Acta Crystallogr. A 46, 467.
G. M. Sheldrick (2008). Acta Crystallogr. A 64, 112.
G. M. Sheldrick (2015). Acta Crystallogr. C 71, 3.
G. M. Sheldrick (2015). Acta Crystallogr. A 71, 3.
C. B. Hübschle, G. M. Sheldrick, and B. Dittrich (2011). J. Appl. Crystallogr. 44, 1281.
K. Brandenburg, H. Putz, and DIAMOND, Crystal Impact GbR (Bonn, Germany, 2019).
A. Simon, H.-G. von Schnering, and H. Schäfer (1967). Z. Anorg. Allg. Chem. 355, 295.
Acknowledgements
The authors thank Dr. A. Villinger (Universität Rostock) for taking care of the X-ray facilities. Financial support from the Deutsche Forschungsgemeinschaft through the SPP 1708 is gratefully acknowledged (KO 1616/8-1 and2).
Funding
Open Access funding enabled and organized by Projekt DEAL. Funding was provided by Deutsche Forschungsgemeinschaft (KO1616/8-1/2).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schröder, F., Köckerling, M. Alcoholate and Mixed Alcoholate/Iodide Supported Hexanuclear Niobium Cluster Compounds with a Mixed Face-Bridged/Edge-Bridged Cluster Pair Example. J Clust Sci 34, 1413–1421 (2023). https://doi.org/10.1007/s10876-022-02300-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-022-02300-y