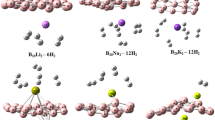
Abstract
Hydrogen is clean energy source that can replace fossil fuels, considering the great need for clean and environmentally friendly energy. Researchers are busy developing new materials for efficient hydrogen storage. Herein, we present the detailed analysis of hydrogen adsorption on pristine B12N12 as well as alkali metals (Li, Na and K) centered B12N12. B3LYP/6-31G(d,p) basis set of DFT has been used in this investigation. These parameters are carried out to analyze the structure, stability, and reactivity of centered nano-cage towards hydrogen. Firstly, we optimized alkali metals (Li, N,a and K) centered nanocage. And then these nano-clusters are analyzed for hydrogen adsorption. Adsorption energies, bond lengths, HOMO–LUMO gap, molecular electrostatic potential, charge density, and PDOS analysis are performed by using the B3LYP/6-31(d,p) basis set of DFT. All centered nano-cages offer better adsorption of hydrogen as compared to pure B12N12. Dipole moment analysis indicates that a high charge density exists when H2 is adsorbed on metals centered in B12N12. MEP shows that charge separation occurred when hydrogen is adsorbed on a metals-centered nano-cage. HOMO–LUMO energy gap investigation shows that LUMO shows stabilization while HOMO is destabilized when metal or hydrogen are in contact with BN nano-cage. Alkali metals encapsulation can improve the chemical and physical properties of B12N12. A novel type of system for developing hydrogen storage materials was finally proposed.
Graphical Abstract





Similar content being viewed by others
References
J. Beheshtian, M. Kamfiroozi, Z. Bagheri, and A. Ahmadi (2012). Computat. Mater. Sci. 54, 115.
L. Schlapbach, A. Zütte (2011) World Sci. 265.
M. Dresselhaus and I. Thomas (2001). Nature 414, 332.
L. Schlapbach and A. Züttel (2001). Nature 414, 353.
J. Beheshtian and I. Ravaei (2016). Appl. Surf. Sci. 368, 76.
K. Chandrakumar and S. K. Ghosh (2008). Nano Lett. 8, 13.
K. Gopalsamy and V. Subramanian (2014). Int. J. Hydrog. Energy 39, 2549.
X. Li, S. Wang, Y. Zhu, G. Yang, and P. Zheng (2015). Int. J. Hydrog. Energy 40, 330.
J. P. B. Ramirez, D. Halm, J.-C. Grandidier, and S. Villalonga (2015). Int. J. Hydrog. Energy 40, 13165.
R. Loisel, L. Baranger, N. Chemouri, S. Spinu, and S. Pardo (2015). Int. J. Hydrog. Energy 40, 6727.
M. Ismail (2016). Mater. Today 3, S80.
J. A. Ritter, A. D. Ebner, J. Wang, and R. Zidan (2003). Mater. Today 6, 18.
F. Schüth, B. Bogdanovi, and M. Felderhoff (2004) Chem. Commun. 2249.
A. Lale, S. Bernard, and U. B. Demirci (2018). ChemPlusChem 83, 888.
K. Kalateh, G. A. Cordshooli, and S. Kheirollahpoor (2017). Nanotubes Carbon Nanostruct. 25, 459.
W.-J. Xu, Z.-Y. Hu, and X.-H. Shao (2012). Acta Physico-Chimica Sinica 28, 1721.
W. Andreoni, A. Curioni, J. M. Kroes, F. Pietrucci, and O. Gröning (2012). J. Phys. Chem. C 116, 269.
Z. Li, G. Zhu, G. Lu, S. Qiu, and X. Yao (2010). J. Am. Chem. Soc. 132, 1490.
J. Iqbal, R. Ludwig, and K. Ayub (2017). Mater. Res. Bull. 92, 113.
S. Wen, W. Deng, K. Han, and B. Endohedral (2008). J. Phys. Chem. C 112, 12195.
N. Karachi and A. Boshra (2018). Heteroatom Chem. 29, e21435.
M. Moral, J. M. Granadino-Roldán, A. Garzón, G. García, and M. Fernández-Gómez (2011). Chem. Phys. 379, 51.
E. Tahmasebi, E. Shakerzadeh, and Z. Biglari (2016). Appl. Surf. Sci. 363, 197.
A. New (2008). Nano Lett. 8, 3166.
N. S. Venkataramanan, R. V. Belosludov, R. Note, R. Sahara, H. Mizuseki, and Y. Kawazoe (2010). Chem. Phys. 377, 54.
M. Valetas, M. Verite, A. Bessaudou, F. Cosset, and J. Vareille (2005). Comput. Mater. Sci. 33, 163.
L. Zhou and S.-Q. Shi (2002). Comput. Mater. Sci. 23, 166.
R. T. Paine and C. K. Narula (1990). Chem. Rev. 90, 73.
T. Oku, T. Hirano, M. Kuno, T. Kusunose, K. Niihara, and K. Suganuma (2000). Mater. Sci. Eng. B 74, 206.
S. Homeyer and W. Sachtler, Design of Metal Clusters in Nay Zeolite, Studies in Surface Science and Catalysis. (Elsevier, Amsterdam, 1989), pp. 975–984.
H. Haberland, Clusters of Atoms and Molecules: Theory, Experiment, and Clusters of Atoms (Springer, New York, 2013).
B. K. Rao, P. Jena, S. Burkart, G. Ganteför, and G. Seifert (2001). Phys. Rev. Lett. 86, 692.
S. K. Yadav and J. W. Cho (2013). Appl. Surf. Sci. 266, 360.
E. Shakerzadeh, E. Tahmasebi, and Z. Biglari (2016). J. Mol. Liquids 221, 443.
M. Eslami, V. Vahabi, and A. A. Peyghan (2016). Physica E 76, 6.
S. Jia, Z. Wang, N. Ding, Y.-L.E. Wong, X. Chen, G. Qiu, and T.-W.D. Chan (2016). Anal. Chim. Acta 936, 123.
J. Beheshtian, M. B. Tabar, Z. Bagheri, and A. A. Peyghan (2013). J. Mol. Modeling 19, 1445.
A. A. Peyghan, S. A. Aslanzadeh, and A. Samiei (2014). Monatshefte für Chemie-Chemical Monthly 145, 1083.
A. V. Moradi, A. A. Peyghan, S. Hashemian, and M. T. Baei (2012). Bull. Korean Chem. Soc. 33, 3285.
A. Ahmadi, J. Beheshtian, and N. L. Hadipour (2011). Physica E 43, 1717.
J. Beheshtian, M. Kamfiroozi, Z. Bagheri, and A. A. Peyghan (2012). Chin. J. Chem. Phys. 25, 60.
M. T. Baei, A. A. Peyghan, and Z. Bagheri (2012). Bull. Korean Chem. Soc. 33, 3338.
M. D. Esrafili and R. Nurazar (2014). Surf. Sci. 626, 44.
J. Beheshtian, A. A. Peyghan, Z. Bagheri, and M. Kamfiroozi (2012). Struct. Chem. 23, 1567.
A. K. Kandalam, M. Blanco, and R. Pandey (2001). J. Phys. Chem. B 105, 6080.
M. Frisch, G. Trucks, H. Schlegel, G. Scuseria, M. Robb, J. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G.A. Petersson et al. Gaussian09, Rev. C. 01 (Gaussian, Inc., Wallingford, 2010).
A. Soltani, M. T. Baei, M. R. Taghartapeh, E. T. Lemeski, and S. Shojaee (2015). Struct. Chem. 26, 685.
A. S. Rad, Y. M. Jouibary, V. P. Foukolaei, and E. Binaeian (2016). Curr. Appl. Phys. 16, 527.
A. S. Rad and V. P. Foukolaei (2015). Synth. Met. 210, 171.
F. Jensen and H. Toftlund (1993). Chem. Phys. Lett. 201, 89.
L.-H. Gan and J.-Q. Zhao (2009). Physica E 41, 1249.
R. G. Parr, L. V. Szentpály, and S. Liu (1999). J. Am. Chem. Soc. 121, 1922.
T. Koopmans (1933). Physica 1, 104.
R. G. Parr and W. Yang, Density-functional theory of atoms and molecules International Series of Monographs on Chemistry, vol. 3. (Oxford University Press, New York, 1994), pp. 14312–14321.
A. Bongini, M. Panunzio, G. Piersanti, E. Bandini, G. Martelli, G. Spunta, and A. Venturini (2000). Eur. J. Org. Chem. 2000, 2379.
T. Lu, Multiwfn: A Multifunctional Wave Function Analyzer, version 3.3. 7; (2015).
S. S. Li, Energy Band Theory, Semiconductor Physical Electronics. (Springer, New York, 2006), pp. 61–104.
G. Shi, Y. Wang, F. Zhang, B. Zhang, Z. Yang, X. Hou, S. Pan, and K. R. Poeppelmeier (2017). J. Am. Chem. Soc. 139, 10645.
M. Mutailipu, M. Zhang, B. Zhang, L. Wang, Z. Yang, X. Zhou, and S. Pan (2018). Angew. Chem. Int. Ed. 57, 6095.
M. Mutailipu, K. R. Poeppelmeier*, and S. Pan* (2021) Chem. Rev. 121, 1130.
M. Mutailipu, Z. Xie, X. Su, M. Zhang, Y. Wang, Z. Yang, M. R. S. A. Janjua, and S. Pan (2017). J. Am. Chem. Soc. 139, 18397.
A. Mahmood (2019). J. Clust. Sci. 30, 1123.
A. Mahmood, A. Tang, X. Wang, and E. Zhou (2019). Phys. Chem. Chem. Phys. 21, 2128.
A. Mahmood, S.U.-D. Khan, and U. A. Rana (2014). J. Comput. Electron. 13, 1033.
A. Mahmood, J. Yang, J. Hu, X. Wang, A. Tang, Y. Geng, Q. Zeng, and E. Zhou (2018). J. Phys. Chem. C 122, 29122.
A. Mahmood, M. I. Abdullah, and S.U.-D. Khan (2015). Spectrochim. Acta Part A 139, 425.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declared no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mehboob, M.Y., Hussain, R., Younas, F. et al. Computation Assisted Design and Prediction of Alkali-Metal-Centered B12N12 Nanoclusters for Efficient H2 Adsorption: New Hydrogen Storage Materials. J Clust Sci 34, 1237–1247 (2023). https://doi.org/10.1007/s10876-022-02294-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-022-02294-7