Abstract
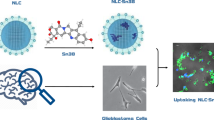
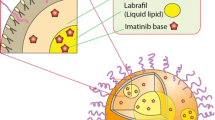
In the present study, orally administered Osimertinib nanostructured lipid carriers (OSM-NLCs) was prepared by the hot-melt emulsification and sonication method. The formulations were optimized by Box-Behnken design to get the optimum composition. The optimized formulation (OSM-NLCop) was further evaluated for in-vitro, ex-vivo parameters as well as in-vitro cytotoxicity evaluation against lung cancer cell line. The selected OSM-NLCop has shown a particle size of 162.6 ± 3.5 nm, PDI 0.25, entrapment efficiency 80.2 ± 3.9% with the negative zeta potential (− 24.7 mV). OSM-NLCop showed significant (p < 0.05) high drug release (82.52 ± 5.4%) as compared to pure OSM (24.7 ± 3.6%). Drug release profile from OSM-NLCop displayed Fickian release kinetic with Korsmeyer-Peppas release model. It also exhibited significantly high permeation with 4.8-fold enhancement in the permeation flux than pure OSM dispersion. OSM-NLCs also depicted (p < 0.05) lesser cytotoxicity against the tested lung cancer cell and reduced IC50 value as compared to pure OSM. From the findings, it can be concluded that NLCs are promising carriers for the delivery of OSM and may improve the therapeutic efficacy against lung cancer cell lines.








Similar content being viewed by others
References
R. L. Siegel, K. D. Miller, and A. Jemal (2018). Cancer statistics, 2018. CA: Cancer J. Clin. 68, 7–30.
J. Zhu, Y. Huang, J. Zhang, Y. Feng, and L. Shen (2020). Formulation, preparation and evaluation of nanostructured lipid carrier containing naringin and coix seed oil for anti-tumor application based on “unification of medicines and excipients.” Drug Des Devel Ther. 14, 1481–1491.
P. Zhou, G. Chen, M. Gao, and J. Wu (2018). Design, synthesis and evaluation of the osimertinib analogue (C-005) as potent EGFR inhibitor against NSCLC. Bioorgan. Med. Chem. 26 (23–24), 6135–6145.
S. S. Ramalingam, J. Vansteenkiste, D. Planchard, B. C. Cho, J. E. Gray, Y. Ohe, C. Zhou, T. Reungwetwattana, Y. Cheng, B. Chewaskulyong, R. Shah, M. Cobo, K. H. Lee, P. Cheema, M. Tiseo, T. John, M. C. Lin, F. Imamura, T. Kurata, A. Todd, R. Hodge, M. Saggese, Y. Rukazenkov, and J. C. Soria (2020). Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N. Engl. J. Med. 382, 41–50.
N. Kommineni, E. Nottingham, A. Bagde, N. Patel, A. Rishi, S. R. S. Dev, and M. Singh (2021). Role of nano-lipid formulation of CARP-1 mimetic, CFM-4.17 to improve systemic exposure and response in osimertinib resistant non-small cell lung cancer. Eur J Pharm Biopharm. 158, 172–184.
S. S. Sawant, S. M. Patil, S. K. Shukla, N. S. Kulkarni, V. Gupta, and N. K. Kunda (2021). Pulmonary delivery of osimertinib liposomes for non-small cell lung cancer treatment: formulation development and in vitro evaluation. Drug Deliv and Transl Res. https://doi.org/10.1007/s13346-021-01088-0.
M. K. Anwer, M. M. Ahmed, F. Fatima, S. M. Alshahrani, M. F. Aldawsari, A. Alalaiwe, R. Al-Shdefat, and F. Shakeel (2019). Solubility, solution thermodynamics and molecular interactions of osimertinib in some pharmaceutically useful solvents. J. Mol. Liq 284, 53–58.
M. M. Ahmed, F. Fatima, M. K. Anwer, M. F. Aldawsari, B. K. Almutairy, S. M. Alshahrani, M. Iqbal, S. Bhatia, and A. Z. Alanazi (2020). Development and characterization of osimertinib loaded ethylcellulose based sustained release nanosponges: cytotoxicity and biocompatibility studies. Lat. Am. J. Pharm. 39 (7), 1292–1299.
P. Skupin-Mrugalska and T. Minko (2020). Development of liposomal vesicles for osimertinib delivery to EGFR mutation-positive lung cancer cells. Pharmaceutics. 12 (10), 939.
X. Hu, S. Chen, H. Yin, Q. Wang, Y. Duan, L. Jiang, and L. Zhao (2020). Chitooligosaccharides-modified PLGA nanoparticles enhance the antitumor efficacy of AZD9291 (Osimertinib) by promoting apoptosis. Int. J. Biol. Macromol. 162, 262–272.
M. H. Kim, K. T. Kim, S. Y. Sohn, J. Y. Lee, C. H. Lee, H. Yang, B. K. Lee, K. W. Lee, and D. D. Kim (2019). Formulation and evaluation of nanostructured lipid carriers (NLCs) Of 20(S)-protopanaxadiol (PPD) by box-behnken design. Int. J. Nanomed. 14, 8509–8520.
T. Ai, W. Shang, H. Yan, C. Zeng, K. Wang, Y. Gao, T. Guan, C. Fang, and J. Tian (2018). Near infrared-emitting persistent luminescent nanoparticles for hepatocellular carcinoma imaging and luminescence-guided surgery. Biomaterials. 167, 216–225.
K. C. Bentz and D. A. Savin (2018). Hollow polymer nanocapsules: synthesis, properties, and applications. Polym. Chem. 9, 2059–2081.
C. Thapa, A. Ahad, M. Aqil, S. S. Imam, and Y. Sultana (2018). Formulation and optimization of nanostructured lipid carriers to enhance oral bioavailability of telmisartan using Box-Behnken design. J. Drug Del. Sci. Tech. 44, 431–439.
R. H. Muller, M. Radtke, and S. A. Wissing (2002). Solid lipid nanoparticles (sln) and nanostructured lipid carriers (nlc) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 54 (Suppl 1), S131-155.
S. M. Moghddam, A. Ahad, M. Aqil, S. S. Imam, and Y. Sultana (2016). Optimization of nanostructured lipid carriers for topical delivery of nimesulide using box-behnken design approach. Artif. Cells Nanomed. Biotechnol. 45(3), 617–624.
E. B. Souto, S. A. Wissing, C. M. Barbosa, and R. H. Muller (2004). Development of a controlled release formulation based on sln and nlc for topical clotrimazole delivery. Int. J. Pharm. 278, 71–77.
V. Jenning, A. F. Thunemann, and S. H. Gohla (2000). Characterisation of a novel solid lipid nanoparticle carrier system based on binary mixtures of liquid and solid lipids. Int. J. Pharm. 199, 167–177.
C. Y. Chen, Y. H. Lee, S. H. Chang, Y. F. Tsai, J. Y. Fang, and T. L. Hwang (2019). Oleic acid-loaded nanostructured lipid carrier inhibit neutrophil activities in the presence of albumin and alleviates skin inflammation. Int. J. Nanomed. 14, 6539–6553.
O. A. A. Ahmed, U. A. Fahmy, R. Bakhaidar, et al. (2020). Pumpkin oil-based nanostructured lipid carrier system for antiulcer effect in NSAID-induced gastric ulcer model in rats. Int. J. Nanomed. 15, 2529–2539.
G. Shevalkar, M. Pawar, and P. Vavia (2021). Nanostructured lipid carriers (NLCs) of lumefantrine with enhanced permeation. J Pharm Innov. https://doi.org/10.1007/s12247-021-09590-1.
X. Peng, G. Yang, Y. Shi, et al. (2020). Box-Behnken design based statistical modeling for the extraction and physicochemical properties of pectin from sunflower heads and the comparison with commercial low-methoxyl pectin. Sci. Rep. 10, 3595.
A. Kumar, A. Singh, S. J. S. Flora, and R. Shukla (2021). Box-behnken design optimized TPGS coated bovine serum albumin nanoparticles loaded with anastrozole. Curr. Drug Deliv. 18 (8), 1136–1147.
D. Parashar, N. P. Aditya, and R. S. R. Murthy (2016). Development of artemether and lumefantrine co-loaded nanostructured lipid carriers: physicochemical characterization and in vivo antimalarial activity. Drug Deliv. 23, 123–129.
M. N. Saleem and M. Idris M (2016). Formulation design and development of a unani transdermal patch for antiemetic therapy and its pharmaceutical evaluation. Scientifica Article ID 7602347.
J. M. Haigh and E. W. Smith (1994). The selection and use of natural and synthetic membranes for in vitro diffusion experiments. Eur J. Pharm. Sci. 2 (5–6), 311–330.
M. J. Ansari and S. M. Alshahrani (2021). Solubility determination of osimertinib mesylate in various solvents by validated, rapid, highly sensitive, water rich reverse phase high-performance liquid chromatography. Lat. Am. J. Pharm. 40 (5), 1084–1093.
T. Mosmann (1983). Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Imm Meth. 65, 55–63.
L. Tolosa, M. T. Donato, and M. J. Gómez-Lechón (2014). General cytotoxicity assessment by means of the MTT assay. Protocols in In Vitro Hepatocyte Res 333–348.
I. Jazuli, B. Nabi, T. Moolakkadath, T. Alam, S. Baboota, and J. Ali (2019). Optimization of nanostructured lipid carriers of lurasidone hydrochloride using box-behnken design for brain targeting: in vitro and in vivo studies. J. Pharm. Sci. 108, 3082–3090.
T. Alam, S. Khan, B. Gaba, F. Haider, S. Baboota, and J. Ali (2018). Adaptation of quality by design-based development of isradipine nanostructured-lipid carrier and its evaluation for in vitro gut permeation and in vivo solubilization fate. J. Pharm. Sci. 107 (11), 2914–2926.
N. Rangaraj, S. R. Pailla, S. Shah, S. Prajapati, and S. Sampathi (2020). QbD aided development of ibrutinib-loaded nanostructured lipid carriers aimed for lymphatic targeting: evaluation using chylomicron flow blocking approach. Drug Del. Trans. Res. 10, 1476–1494.
S. Uprit, R. Kumar Sahu, A. Roy, et al. (2013). Preparation and characterization of minoxidil loaded nanostructured lipid carrier gel for effective treatment of alopecia. Saudi Pharm J. 21 (4), 379–385.
M. Elmowafy, H. M. Ibrahim, M. A. Ahmed, K. Shalaby, A. Salama, and H. Hefesha (2017). Atorvastatin-loaded nanostructured lipid carriers (NLCs): strategy to overcome oral delivery drawbacks. Drug Deliv. 24 (1), 932–941.
R. S. Rabelo, I. F. Oliveira, V. M. da Silva, A. S. Prata, and M. D. Hubinger (2018). Chitosan coated nanostructured lipid carriers (NLCs) for loading Vitamin D: a physical stability study. Int. J. Biol. Macromol. 119, 902–912.
M. Danaei, M. Dehghankhold, S. Ataei, D. F. Hasanzadeh, R. Javanmard, A. Dokhani, S. Khorasani, and M. R. Mozafari (2018). Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 10 (2), 57.
Joseph E, Singhvi G (2019) Multifunctional nanocrystals for cancer therapy: a potential nanocarrier. Nanomat. Drug Del. Ther. 91–116
P. W. Dhore, V. S. Dave, S. D. Saoji, Y. S. Bobde, C. Mack, and N. A. Raut (2017). Enhancement of the aqueous solubility and permeability of a poorly water soluble drug ritonavir via lyophilized milk-based solid dispersions. Pharm. Dev. Technol. 22 (1), 90–102.
W. Wu, Y. Zu, L. Wang, L. Wang, H. Wang, Y. Li, M. Wu, X. Zhao, and Y. Fu (2017). Preparation, characterization and antitumor activity evaluation of apigenin nanoparticles by the liquid antisolvent precipitation technique. Drug Del. 24 (1), 1713–1720.
S. Shanmugam, C. K. Song, S. N. Sriraman, R. Baskaran, C. S. Yong, H. G. Choi, D. D. Kim, J. S. Woo, and B. K. Yoo (2009). Physicochemical characterization and skin permeation of liposome formulations containing clindamycin phosphate. Arch Pharm. Res. 32 (7), 1067–1075.
Y. Neupane, M. Srivastava, N. Ahmad, N. Kumar, A. Bhatnagar, and K. Kohli (2014). Lipid based nanocarrier system for the potential oral delivery of decitabine: Formulation design, characterization, ex vivo, and in vivo assessment. Int. J. Pharm. 477 (1–2), 601–612.
A. Khosa, S. Reddi, and R. N. Sahab (2018). Nanostructured lipid carriers for site-specific drug delivery. Biomed. Pharmacother. 103, 598–613.
Acknowledgement
This research project is supported by Princess Nourah bint Abdulrahman University Researcher Supporting Project number (PNURSP2022R108), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia for supporting this project.
Funding
Authors are thankful to Princess Nourah bint Abdulrahman University Researcher Supporting Project Number (PNURSP2022R108), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia for funding this research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gilani, S.J., Bin-Jumah, M.N., Imam, S.S. et al. Formulation of Osimertinib Nano Lipid Carriers: Optimization, Characterization and Cytotoxicity Assessment. J Clust Sci 34, 1051–1063 (2023). https://doi.org/10.1007/s10876-022-02282-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-022-02282-x