Abstract
Decreasing the salivary flow rate manifested by xerostomia occurs early during the irradiation treatment. The duration of depressed salivary function varies among patients. Various histopathological changes occur in the salivary glands. The current study was performed to investigate and compare between the possible anti-radiotherapeutic effect of the gamma rays-synthesized curcumin nanoparticles (Cur NPs), and chitosan nanoparticles (Cs NPs). They were utilized to overcome the histopathological changes associated with radiation therapy in albino rats’ parotid glands. Sixty adult male Albino rats were utilized, fifteen as control group, fifteen as radiated group and thirty as Cur NPs and Cs NPs treatment groups. The parotid glands were dissected and examined histologically, immunohistochemically for vascular endothelial growth factor (VEGF) as well as histo-morphometrically. The histological results proved the antiradio-therapeutic effect of Cur NPs, and Cs NPs, with the least degenerative changes in the Cur NPs treated group. A high significant increase in VEGF was recorded in the radiated group as compared to the other treated groups. Cs NPs have proved to be an anti-radiotherapeutic and anti-oxidant substrate in treating the histopathological changes in radiated parotid glands of albino rats. However, it was lagging behind Cur NPs in all analyses but non-significant differences between them have been recorded.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is still the leading cause of mortality worldwide. According to the International Agency for Research on Cancer (IARC), 7.6 million people die each year as a result of cancer [1]. The use of radiotherapy for cancer treatment varies greatly across the globe. It has been suggested that about half of all cancer patients should receive radiation treatment [2]. Early periods in the irradiation treatment, many adverse effects such as decreased salivary flow rate and mouth dryness develop [3]. Irradiation at high dosages breaks double-stranded DNA in tumor cells, causing them to die. On the other hand, inappropriate amounts of irradiation cause tumor cells to emit damage-associated molecular pattern molecules, cytokines and reactive oxygen species, which may interact with immune cells [4]. Anti-oxidants are generally reducing substances such as thiols, ascorbic acid or polyphenols, since they block the oxidation of other molecules by oxidizing themselves [5].
Chitosan is found in shellfish, clams, krill, oysters, squid, mushrooms and insects and is one of the most abundant naturally occurring polysaccharides [6]. It contains antioxidant and membrane-stabilizing capabilities, according to reports. Chitosan's antiulcerogenic ability against HCl–ethanol-induced peptic ulcer in rats was demonstrated [7].
Curcumin (diferuloylmethane), derived from the turmeric plant Curcuma longa and its derivatives known as curcuminoids, has been studied as an anti-inflammatory agent for cancer and other disorders [8]. The creation of nano-range formulations of curcumin, often known as "nano-curcumin", has provided solid proof that this agent can reap all of its biological benefits [9]. Curcumin has been suggested as a possible way to shield normal cells from the detrimental effects of radiation. Its capacity to lower oxidative stress and suppress transcription of genes related to oxidative stresses and inflammatory responses may be the fundamental reason for its radioprotective activity [10, 11]. The expression of the inflammatory cytokines NFkB, IL6, COX1, and COX2 mRNA was significantly reduced when the cells were treated with Cur NPs. These findings suggest that Cur NPs could be a promising anti-inflammatory medication [12].
The novelty of current study is to investigate and compare the possible anti-radiotherapeutic effect of the synthesized Cur NPs, and Cs NPs to overcome the histopathological changes associated with radiation therapy in albino rats’ parotid glands. The histological results proved the antiradio-therapeutic effect of Cur NPs, and Cs NPs, with the least degenerative changes in the Cur NPs-treated group. Demonstrating the anti-radiotherapeutic role of Cur NPs was the main rational of the present research, and was achieved by studying the histological, histo-morphometric and immune-histochemical results of anti-VEGF anti-body.
Materials and Methods
Materials
Chitosn powder (Mw, 750,000 Da; viscosity, 8 cP; deacetylation rate, 85–87%; purity, 98.6%) was procured from M/s Sigma Chemical Company. Curcumin; Turmeric curcumin powder as pure nature 100% (Standardized to 95% Curcuminoids) was procured from Now Food Company.
Synthesis of Cs NPs and Cur NPs
Firstly chitosan (2 g) was dissolved in acetic acid (2%, 100 ml) for 60 min at room Temp., and curcumin was dissolved in distilled water (100 ml), we try the age old technique of heating the mixture of the curcumin and water, up to 70–80 °C for 60 min according to Ramya Jagannathan et al. [13]; boiling will not harm the molecule though. Finally, to get a homogenous solution of curcumin in water, filtration of the heterogeneous mixture was performed.
All the solutions were mixed with 2 ml of isopropanol to prevent additional free radical, and all solutions were gamma-irradiated at 15 kGy. The color changes were due to the surface Plasmon resonance (SPR) and confirmed the formation of Cs NPs, and Cur NPs [14,15,16,17]. The color was observed to be milky (off-white) in case of Cs NPs [18], and yellow for Cur NPs [14, 19]. Figure 1 illustrated the graphical representation regarding the preparation method for Cs NPs, and Cur NPs.
Characterization of the Synthesized Cs NPs and Cur NPs
Different techniques were used for characterizing the prepared Cs NPs and Cur NPs. The shape and size of the prepared samples were studied using a high-resolution transmission electron microscope (HRTEM, JEM2100, JEOL, Japan) while, the surface structure and homogeneity of the synthesized samples were characterized by scanning electron microscopy (SEM) ZEISS, EVO-MA10. The zeta potential, surface charge, polydispersity index (PDI) and particle size distribution in suspension of Cs NPs and Cur NPs were measured by a dynamic light scattering Zeta-sizer (Malvern 3000HSa, UK).
Animal Model
Sixty adult male Albino rats with an average weight 200–250 g, were obtained from the animal house, Atomic Energy Authority. The animals were housed in controlled environment (Temperature 25 ± 2 °C and 12 h dark/light cycles) and fed with standard pellets diet and tap water adlibitum. They were kept in individual cages. All experiments were conducted in the animal house in National Center for Radiation Research and technology, Egypt according to the recommendations of the ethics committee on animal’s experimentation of the Faculty of Oral and Dental Medicine, Minia University (ethical approval number 399/2020).
Animal grouping; the animals were divided in to 4 main groups. Group I (control group) which consisted of 15 healthy rats away from the radiation building. Group II (radiated group) that consisted of 15 rats. Rats were arranged in lead cylinder to protect the rest of body from radiation, while heads were exposed to radiation. Radiation was done using cobalt60 radioisotope source [energy 1.25 MV (millionvolts)]. The rate of dose is 0.971 K.Gy/h (0.2697 Gy/sec), Indian gamma Cell. The rats' heads were irradiated to a dose of 15 Gy (56 s) given as a single shot at SSD (source surface distance) 80 cm [20]. Group III (Cur NPs-treated group) consisted of 15 rats, each received a daily Cu NPs which was diluted by distilled water (1: 8) [21], was administered by oral gavage before radiation by 48 h then 24 h then every day for one week (100 mg/kg body weight per day) [22]. Group IV (Cs NPs-treated group) consisted of 15 rats, each were received Cs NPs (200 mg/kg body weight per day, MW 3,80,000 Da); chitosan 0.3% (w/v) of chitosan was dissolved in 2% (v/v) of acetic acid to form chitosan solution [23]. It will be administered by oral gavage before radiation by 48 h then 24 h then every day for one week [24].
The animals were slain by an intracardiac injection of ketamin overdose once the dose was completed and the parotid gland was dissected [25]. The tissues were washed in saline solution and fixed for 24 h in 4 percent buffered formalin, then dehydrated in ethyl alcohol in successive stages, cleaned in xylene and embedded in paraffin. On positively charged microscope slides, 4–5 m thick sections were obtained and collected. Before histological staining and immune-labeling, tissue sections were deparaffinized and rehydrated. There are some controls missing like the administration of curcumin in solution and chitosan in solution to prove that their nano-sized organization was responsible by the preventive effect.
Irradiation Process
The animals were weighed and sedated with an intramuscular dose of thiopental 70 mg/kg and atropine 40 mg/kg 30 min before the irradiations [26]. Animals were placed on the cylinder lead to protect the body, so that only the head and neck regions were exposed, and the rate of dose is about 26.97 cGy/sec (Fig. S1). The rats' heads were irradiated to a dose of 15 Gy given as a single shot at SSD source surface distance) 80 cm. The 100% of the dose of this radiation source is expected to be at 0.5 cm from the skin [20].
Tissue Samples
After completion of the experiment period, the animals were sacrificed with intra-peritoneal injection of ketamine at 100 mg/kg and the parotid glands were dissected. The tissues were washed in saline solution and fixed for 24 h in 4 percent buffered formalin, then dehydrated in ethyl alcohol in successive stages, cleaned in xylene, and embedded in paraffin. On positively charged microscope slides, 4–5 m thick sections were obtained and collected. Before histological staining and immunolabeling, tissue sections were deparaffinized and rehydrated (Fig. S2).
Haematoxylin and Eosin (H&E) Staining
For histological evaluation of morphological alterations, a series of sections from each of the four groups was stained with haematoxylin and eosin solutions, dried, mounted and inspected in a Leica light microscope equipped with a digital camera and image processing software.
Immunohistochemistry
De-paraffinization and dehydration were performed on the sections. Endogenous peroxidase activity was inhibited for 10 min at room temperature using a 3 percent hydrogen peroxide solution. After that, they were rinsed in phosphate buffered saline (PBS) and immersed for 10–20 min in antigen retrieval solution (10 mM citrate buffer, PH 6.0). Slides were incubated in the ready-to-use pre-diluted primary VEGF antibody (Thermo Fisher Scientific Inc., UK. Cat. #RB-9034-R7) for 2 h at room temperature before being rinsed in PBS. Incubation in biotinylated secondary antibody for 10–15 min at room temperature was performed. After rinsing with PBS, incubation with streptavidin peroxidase for another 10–15 min at room temperature. Then, rinsing with PBS and incubation with 3, 3′-diaminobenzidine (DAB) substrate chromogen at room temperature for 10 min. Followed by washing in PBS, the slides were immersed in haematoxylin bath for 30 s-1 min, and then washed in water. The slides were left to dry in air, then mounted in Canada balsam. All immune-histochemical staining were done in a Dako Auto-Stainer under the same conditions.
Histo-morphometry Analyses
The image analyzer computer system consisted of a digital camera attached to a light microscope and a computer system equipped with the software Leica Quin 5000 (Leica Microsystems Inc., Switzerland) capable of performing high speed digital image processing. H&E-stained sections were used to count the number of nuclei per field and the number of acini per field using magnification (400×). In the Immuno-labelled sections for VEGF the area and area percentage of VEGF immunoreaction in 15 fields from each group using magnification (400×) were measured.
Statistical Analysis
The statistical analysis was performed using the arithmetic mean (X), standard deviation (SD), and analysis of variance (ANOVA). All statistical analyses were done on an IBM PC using the statistical software "SPSS 17" (Statistical Package for Scientific Studies) for Windows. Results were considered significant when probability (P-) value was ≤ 0.05, highly significant when P-value ≤ 0.01 and very highly significant when P-value ≤ 0.001.
Results
Characterization of the Synthesized Cs NPs and Cur NPs
As shown in Fig. 2, the synthesized Cs NPs and Cur NPs were first analyzed using HRTEM. As illustrated in Fig. 2A, typical thin layer in the shape of a spherical particle was observed, confirming the produced Cs NPs. The particle sizes ranged from 25.0 to 29.0 nm, with an average particle size of 27.40 nm. Figure 2B shows the HRTEM image of Cur NPs, which showed a well-defined rounded particle and mono-dispersed NPs with sizes ranging from 13.5 to 23.48 nm and an average particle size of 19.95 nm.
The SEM imaging procedure discriminated the external and morphological aspects of the produced Cs NPs and Cur NPs. The fundamental morphology of the integrated Cs NPs retains some aggregated nano-spheres and a mono-dispersed particle, as shown in Fig. 3A. The produced Cur NPs were also disseminated as spherical NPs with some aggregates, as shown in Fig. 3B.
The mean particle size dispersion was determined by the DLS method and was found to be 33.5 nm (Fig. 4A), and 22.6 nm (Fig. 4B) in the synthesized Cs NPs and Cur NPs, respectively. It was stated that DLS size range of the synthesized Cs NPs and Cur NPs was noted to be greater than the HRTEM size. It is because of the DLS was estimated the hydrodynamic radius where the synthesized Cs NPs and Cur NPs were enclosed by the water particles and may the reason of the large sizes of the capped Cs NPs and Cur NPs [27].
The polydispersity index (PDI) can be obtained from instruments that use DLS or determined from electron micrographs. In the obtained PDI values, we found that the PDI value was calculated to be 0.423 in Cs NPs (Fig. 4A), and slightly-increased to be 0.462 in the synthesized Cur NPs (Fig. 4B). International standards organizations (ISOs) have established that PDI values < 0.05 are more common to monodisperse samples, while values > 0.7 are common to a broad size (e.g., polydisperse) distribution of particles [28]. The present values (0.423, and 0.462) indicated that the synthesized NPs (Cs NPs and Cur NPs) were moderate mono-size distributed.
The Zeta potential of the synthesized Cs NPs and Cur NPs was examined to determine the surface charge of the synthesized NPs, which in turn determine the stability, as observed in Fig. 4. From the present results, it is notable that the Zeta potential of the synthesized Cs NPs surface maintains a positive statement (12.4 mV; Fig. 4C), and Cur NPs possesses a negative statement (− 12.5 mV; Fig. 4D) at pH 7 (cultural media pH). The magnitude of the zeta potential is predictive of the colloidal stability [29]. Nanoparticles with Zeta potential values from ± 10 to ± 30 have incipient stability [30], as shown in our synthesized NPs. Dispersions with a low Zeta potential value will eventually aggregate due to the Van Der Waal inter-particle attractions [31].
Histological Examination
Group: A (Control Group)
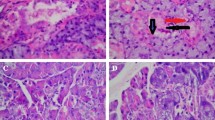
Parotid gland of control group was found to be composed of secretory end pieces and collecting ducts. The secretory end pieces were arranged in spherical fashion and each one was composed of pyramidal shaped serous cells with basally located rounded deeply basophilic nuclei. The intercalated ducts were lined by cuboidal cells and had a narrow and compressed lumens, the striated ducts appeared to be lined by low columnar cells with centrally placed nuclei (Fig. 5A).
photomicrograph of parotid gland group for (A) Group A, where a-normal acini architecture b-normal intercalated duct, and c-normal striated duct, for (B) Group B, where a-abnormal acini architecture, loss af acinar boundry and nuclear hyperchromatism b-dialated and stagnated intercalated duct c-striated duct with entrapped secretion astricks-abnormal nuclear shape and perinuclear haloing, for (C) Group C, where a-acini restored its architecture b-intercalated duct with little dilatation ended by normal striated duct c-dailated blood vessels, and for (D) Group D, where a-acini loss its architecture b-dailated intercalated duct with entrapped secretion c-blood vessel engoraged with RBCs, astricks: nuclei with abnormal shape and size. (H & E, × 400)
Group: B (Radiated Group)
Histological examination and evaluation of hematoxylin and eosin-stained sections of radiated parotid gland in rats revealed increase in the inter acinar and interlobular spaces. The gland appeared to be composed of parenchyma and connective tissue stroma, CT septa thickness was increased in this group. Serous acini were found to lose their architecture and arrangement with ill-defined boundaries. The nuclei of serous acinar cells showed hyperchromatism, pleomorphism, abnormal mitotic figures, karyomegaly and perinuclear haloing. Intercalated ducts appeared to be dilated and stagnated. The lining cells of intercalated ducts appeared with abnormal nuclear morphology. Striated ducts appeared with stagnant secretion, cell proliferation and abnormal nuclear morphology (Fig. 5B).
Group: C (Cur NPs-Treated Group)
Radiated parotid gland treated with Cur NPs showed histological features of normal parotid glands. The acini started to restore its normal architecture. The nuclear changes appeared less than in group B (radiated group). The duct system with less histological deformity (Fig. 5C).
Group: D (Cs NPs-Treated Group)
Administration of Cs NPs to radiated rats revealed some histological changes in their parotid glands that supported its anti-radiotherapeutic hazard’s role to a certain limit. The deterioration in the parotid glands in this group appeared to be less than that noticed in radiated group (Fig. 5D).
Immuno-histochemical of Vascular Endothelial Growth Factor (VEGF)
Group: A (Control Group)
The immunoreaction patterns of VEGF in parotid gland of control rats revealed that secretory cells of serous acini and epithelial lining cells in the intercalated and striated ducts presented negative nuclear and cytoplasmic immunoreaction for VEGF (Fig. 6A).
A Photomicrograph of group (A) showing blue negative reacting nuclei and cytoplasm in a-serous acinar cells b-intercalated duct cells, and c-striated duct cells, B photomicrograph of group (B) showing brown positive reaction of cytoplasm in a-serous acinar cells b-ducts with entrapped secretion, C photomicrograph of group (C) showing weak positive immunoreaction of cytoplasm in a-serous acinar cells b-intercalated duct cells, and c-striated duct cells, and D photomicrograph of group (D) showing moderate positive immunoreaction of cytoplasm in a-serous acinar cells, weak positive immunoreaction in b-intercalated duct cells, and c-striated duct cells. (VEGF, × 400)
Group: B (Radiated Group)
Immuno-stained sections of parotid gland in this group showed that serous acinar cells and duct cells cytoplasm revealed intense immunoreaction for VEGF. On the other hand, nuclei exhibited negative reacting for VEGF (Fig. 6B).
Group: C (Cur NPs-Treated Group)
Examination of the immune-stained sections of parotid gland from group C, incubated with anti VEGF antibody revealed weak immunoreactivity throughout the gland. The serous acinar portions, intercalated duct and striated duct displayed weak cytoplasmic immunoreactivity for VEGF. Negative immunoreactivity in nuclei (Fig. 6C).
Group: D (Cs NPs-Treated Group)
Examination of the immune-stained sections of parotid glands from group D, incubated with anti-VEGF anti-body showed varied immunoreactivity throughout all the gland. The immunoreaction was moderate in serous acinar cell. The duct cells revealed a weak immunoreaction (Fig. 6D).
Histomprphometric and Color Density Area Percentage Statistical Analysis
Statistical analysis revealed a high significant difference between the control group and radiated groups as regarding the number of nuclei/slide, the number of acini/field and color density of VEGF. The difference between radiated group and the Cur NP group was significant regarding acini/field while highly significant regarding nuclei/field and VEGF color density area percentage (Table 1).
The statistical difference between radiated group and Cs NP group was significant regarding acini/slide and nuclei/field, while highly significant regarding color density of VEGF. On the other hand; a non-significant difference has been recorded between Cur NP group and Cs NP group (Table 2).
Discussion
In 2008, the global cancer prevalence was predicted to be 28.8 million people over the age of five. Oral cancer ranks as the most prevalent cancer in Indian men and Kaposi sarcoma has the highest 5-year prevalence among males in eleven countries in Africa [1]. High-dose chemotherapy and radiotherapy have dramatically improved young cancer patients' long-term survival rates, at the cost of significant side effects [32]. To mitigate the adverse effects of radio-therapeutic treatment, there is a need for a chemical or prescription that is naturally available, has no known side effects, and is inexpensive [33]. In this study, Cur NPs, and Cs NPs were tested for their anti-radiotherapeutic effects on the level of histological and structural alterations in the parotid glands of albino rats. A point of weakness in our study that we missed a control group of normal components in comparison to nanosized particles. If we had, it would be strengthening our study more.
We worked on dependence on previous studies. Cur NPs showed significant improvement in kidneys more than curcumin. In contrast, histopathological examination verified the necrosis in negative control group, suggesting the renoprotection effect of Cur NPs against nephrotoxicity on cisplatin-induced rats [34].
Recent study conducted by Sharifi-Rad et al. [35] revealed that, Cs-based nanomaterials showed significant enhancement of drug bioavailability drug loading efficiency, drug-releasing capacity and drug encapsulation efficiency. The latest advantages of Cs NPs applications in nanomedicine are supported also by pre-clinical and clinical studies.
Ionizing radiation causes oxygen-derived free radicals to form in the tissue environment, including hydroxyl radicals (the most harmful), superoxide anion radicals and other oxidants including hydrogen peroxide. Various chemical interactions result in the formation of more damaging radicals [36].
In this study in the radiated group the serous acini appeared irregular in shape and away from each other (increase in inter-acinar spaces). Rupturing of the basement membrane of some acini was revealed. The acinar cells showed variable degrees of atrophy, perinuclear haloing, abnormal mitotic figures, hyperchromatism, karyomegaly and cytoplasmic vacuolization. The nuclei were either elongated and pushed against the cell membrane or binucleated. Extravasated red blood cells (RBCs) were demonstrated between the acini. The ductal epithelial lining revealed thinning and atrophies. Perinuclear haloing and cytoplasmic vacuolization were revealed in all types of ducts. The excretory ducts were detected in between the lobules, surrounded by C.T stroma which revealed hyalinization. The C.T stroma showed increase in thickness and dilatation of blood vessels which were extremely conjugated with RBCs.
All the pervious results agreed with Stramandinoli-Zanicotti et al. [37], who proved that the nuclei of the majority of the cells were prominent, larger and hyperchromatic. In comparison to non-irradiated glands, fibrosis and parenchyma loss were detected. There were numerous ductal alterations, the most prevalent of which was ductal dilation, which was occasionally accompanied by cellular debris.
The perinuclear haloing and cytoplasmic vacuolization demonstrated in the serous acinar cells as well as in the duct cells in the present study could be explained according to Shubin et al. [38], who referred them to the reduction in the water content in the cytoplasm of apoptotic cells. Subsequently, the cells compensate this by swelling and vacuolization. Furthermore, they reported that intense vacuolization that could be lipid filled or autophagic vacuoles led to cell death, either of the lytic or apoptotic type. This could be justified to the increase in the acinar atrophy in IR group in the current work. Another explanation for the perinuclear vacuolization and haloing has been proposed by Short [39], who referred them to the mechanical strain applied on the nuclear membranes that became widely separated. In the present work the increase in the cytoplasmic lipid content could be responsible for that strain.
Binucleated cells detected in the current research are most commonly found in cancer cells. Binucleation has negative effects on cell viability and subsequent mitosis [40]. Taniguchi et al. [41], reported that the cells go through karyokinesis and then cytokinesis in a typical cell division. Some cells, such as cardiomyocytes and hepatocytes, can form binucleated cells without cytokinesis by going through mitosis. The suppression of cytokinesis machinery such as the central spindle or the contractile ring is assumed to be the cause of this cytokinesis skipping, although the mechanisms governing it are unknown.
Acinar atrophy reported in our study explained by Jasmer et al. [42], who proved that, apoptosis of mice salivary acinar cells occurs 8–72 h after irradiation (IR), with the peak occurring at 24 h after IR in the parotid glands. Apoptosis has been measured in a variety of ways to characterize this acute mechanistic state, including increased mRNA expression of apoptosis regulators as well as caspase-3 protein breakage. Salivary flow has been linked to the loss of serous acinar cells, which comprise about 80% of the parotid gland volume and are responsible for water and protein production [42].
In this study; intercalated, striated and excretory duct showed atrophy of cell lining, duct dilatation, entrapped secretion and ductal proliferation these results agreed with Cheng et al. [43], who found that parenchymal loss, acinar atrophy and interstitial fibrosis were found in both irradiated parotid and submandibular glands, as well as duct proliferation, dilated intercalated, and striated duct.
Entrapped or stagnant secretion in ducts might be due to increase saliva viscosity due to change in saliva structure, this could be explained by Winter et al. [44], who proved that after radiation, saliva becomes more viscous, electrolyte levels alter, buffer capacity decreases and the pH shifts from neutral to acidic. Furthermore, important components such as mucoglycoproteins, which influence the viscoelastic nature of saliva and aid lubrication and adherence to the underlying epithelium, showed modifications.
Radiation causes three major events in the tissue as hypocellularity, hypovascularity and hypoxia [45]. Direct cytotoxic effects on acinar cells, as well as ischemia-induced side effects, duct obstruction and a variety of physiological processes that affect degranulation and secretion, are also mentioned. Hypoxia, which can be caused by vascular changes, impairs oxidative aerobic cellular respiration, resulting in cell death [46]. Depending on the severity of the hypoxic state, cells can adapt, suffer injury or even die [47]. The theory of radiation-induced fibrosis proposes that activation and dysregulation of fibroblastic activity cause tissue atrophy in previously irradiated areas. Acute inflammation, free radicals, persistent fibroblast activation and growth factors all cause cell damage [37].
In the ongoing study by calculating the number of nuclei/field, the highest number of nuclei has been detected in the IR group as binucleation predominated. However, by calculating the number of acini/fields a high significant acinar atrophy with radiation was noted. These registrations supported the histological findings in the IR group.
In this study; the signalling protein vascular endothelial growth factor (VEGF) is involved in both vasculogenesis and angiogenesis (the growth of blood vessels from pre-existing vasculature). The effect of radiation on VEGF expression was investigated. Immunohistochemistry of parotid glands from the radiated group revealed high reactivity for VEGF antibody throughout the gland, whereas the control group had no immunoreactivity.
This study findings agreed with Hani et al. [48], who showed an intense positive immunoreactivity to VEGF antibodies in the ducts of parotid gland one-week after radiation, whereas the serous cells showed a moderate to severe reaction. However, there was a moderate immunoreactivity to VEGF in the ductal and most of the acinar cells of the parotid glands of treated group.
Shintani et al. [49], states that, the tumoral vascular network lowered oxygenation and tumor cell sensitivity to radiation, which explained the link between radiation and VEGF. The most common reason of radiation failure is hypoxia. Radiation had a considerable impact on the tumor vascular network, resulting in a decrease in micro vessel density. In addition, the level of VEGF is linked to radiosensitivity and VEGF induction has been seen.
An anti-oxidant is a chemical that prevents other molecules from oxidizing. Oxidation is a chemical reaction in which a material donates electrons or hydrogen to an oxidizing agent. Anti-oxidants are often reducing substances like thiols, ascorbic acids, and polyphenols that do this by becoming oxidized themselves [50]. The present study was performed to compare between the effects of curcumin and chitosan nanoparticles as anti-radiotherapeutic substances.
Curcumin inhibits the generation of nitric oxide (NO), making it a potent anti-tumor agent with anti-inflammatory and anti-oxidant capabilities [51]. Nano-sized biopolymers with improved characteristics have been highlighted as a possible solution in a variety of industries thanks to nanotechnology [52]. Curcumin encapsulation in nanoparticles enhances its circulation inside the body. Nanoparticle-based delivery technologies have emerged to improve the water solubility and raise the bioavailability of medicinal compounds such as curcumin [53]. Demonstrating the anti-radiotherapeutic role of curcumin was the main rational of the present research. This was achieved by studying the histological, histo-morphometric and immune-histochemical results of anti-VEGF anti-body.
In the inherent study, the histological results of the Cur NPs-treated group mimic the histology of normal parotid gland (control group). Serous acini appeared rounded with pyramidal cells and basal basophilic nculei. The acinar atrophy and binucleation appeared less than that demonstrated in the radiated group. These findings are supported with Delavarian et al. [54], who found that the administration of nanocurcumin can be considered as a reasonable approach to hinder the development of oral mucousitis in head and neck cancer patients requiring radiotherapy.
More explanation presented in Liu et al. [55], who explains that, curcumin can reduce chemotherapy-induced toxicity by clearing intracellular reactive oxygen species (ROS) in normal tissues and modulating a number of target molecules including adhesion molecules, inflammatory factors, transcription and growth factors, apoptosis-related proteins and some enzymes and kinases, according to a growing evidence.
Furthermore, in the current work the area percentage of VEGF expression in curcumin nanoparticles treated group showed weaker reaction than radiated group; this agrees with Binion et al. [56], who demonstrated that curcumin inhibits many stages in the angiogenic process by reducing the angiogenic properties of microvascular endothelial cells isolated from the human gut. Curcumin inhibited COX-2 activation as well as continuous production produced by VEGF, according to the researchers.
The other side of comparison is Cs NPs which has been reported to exert antihypertensive, anti-inflammatory, anticoagulant, antitumor, anticancer, antibacterial, hypocholesterolemic, and antidiabetic properties [57].
The parotid glands of Cs NP group in the present study appeared to be as a transitory stage between radiated and Cur NP group. Some acini appeared to preserve its architecture; however acinar fusion appeared in others. Furthermore, binucleation appeared in acini and duct cells. RBCs infusion between acini appeared less than radiated group.
These results agreed with Yang and Young [58], who proved that chitosan promotes salivary gland branching by increasing the expression of basement membrane (BM) components such as collagen, laminin and heparan sulphate proteoglycan, as well as facilitating the expression of BM components and receptors in tissue-specific patterns that are beneficial to salivary gland branching. Chitosan has also been demonstrated to be useful in regulating the development of progenitor salivary tissue.
The correlation between Cs NPs and VEGF has been explained by Huang et al. [59], who reported that short hairpin RNA (shRNA) complexes containing low molecular weight chitosan (LMWC/VEGF) reduced VEGF expression in hepatocellular carcinoma (HCC) cells and liver tumour tissues. When LMWC/shRNA complexes were intravenously delivered into orthotopic allograft liver tumor-bearing mice, the deposition of shRNA at the tumour site was clearly boosted and sustained. In comparison to naked shRNA, intratumoral or intravenous injections of LMWC/VEGF shRNA complexes suppressed tumour angiogenesis and tumour growth more effectively in distinct HCC models.
Conclusion and Future Perspective
In this study, the gamma rays-synthesized Cur NPs, and Cs NPs were tested for their anti-radiotherapeutic effects on the level of histological and structural alterations in the parotid glands of albino rats. Cur NPs, and Cs NPs were found to alleviate histological deformity detected on radiated parotid salivary glands. In histological examination; radiated parotid gland treated with Cur NPs showed histological features of normal parotid glands. The acini started to restore its normal architecture. Administration of Cs NPs to radiated rats revealed some histological changes in their parotid glands that supported its anti-radiotherapeutic hazard’s role to a certain limit. Examination of the immune-stained sections of parotid gland from Cur NPs-treated group, incubated with anti VEGF antibody revealed weak immunoreactivity throughout the gland. On the other hand, the Cs NPs-treated group showed varied immunoreactivity throughout all the gland. There are some controls missing like the administration of curcumin in solution and chitosan in solution to prove that their nano-sized organization was responsible by the preventive effect. Our future work must contains the controls regarding the administration of curcumin in solution/dispersion and chitosan in solution/dispersion.
Data Availability
The authors stated and declare that all data is exist and available.
Code Availability
The authors stated and declare that all code is exist and available.
References
F. Bray, J. S. Ren, E. Masuyer, and J. Ferlay (2013). Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int. J. Cancer 132 (5), 1133–1145.
G. Delaney, S. Jacob, C. Featherstone, and M. Barton (2005). The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 104 (6), 1129–1137.
A. Vissink, F. Burlage, F. Spijkervet, J. Jansma, and R. Coppes (2003). Prevention and treatment of the consequences of head and neck radiotherapy. Crit. Rev. Oral Biol. Med. 14 (3), 213–225.
W. Li, L. Wang, C. Shen, T. Xu, Y. Chu, and C. Hu (2019). Radiation therapy-induced reactive oxygen species specifically eliminates CD19+ IgA+ B cells in nasopharyngeal carcinoma. Cancer Manage. Res. 11, 6299.
R. Fernández-Marín, S. C. Fernandes, C. McReynolds, J. Labidi, and M. Á. A. Sánchez, Chitosan-based materials as templates for essential oils,. Handbook of Chitin and Chitosan. Elsevier, Amsterdam, pp 689–720.
W. M. Brück, J. W. Slater, and B. F. Carney, Chitin and Chitosan from Marine Organisms, Chitin Chitosan, Oligosaccharides and Their Derivatives. (CRC Press, Boca Raton, 2010), pp. 11–23.
R. Anandan, P. V. Nair, and S. Mathew (2004). Anti-ulcerogenic effect of chitin and chitosan on mucosal antioxidant defence system in HCl-ethanol-induced ulcer in rats. J. Pharm. Pharmacol. 56 (2), 265–269.
C. Dende, J. Meena, P. Nagarajan, V. A. Nagaraj, A. K. Panda, and G. Padmanaban (2017). Nanocurcumin is superior to native curcumin in preventing degenerative changes in experimental cerebral malaria. Sci. Rep. 7 (1), 1–12.
G. Flora, D. Gupta, and A. Tiwari (2013). Nanocurcumin: a promising therapeutic advancement over native curcumin, critical reviews™ in therapeutic drug carrier systems. Crit. Rev. Ther. 30 (4), 331.
G. C. Jagetia and B. B. Aggarwal (2007). “Spicing up” of the immune system by curcumin. J. Clin. Immunol. 27 (1), 19–35.
G. C. Jagetia, Radioprotection and radiosensitization by curcumin,. The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease. Springer, Boston, pp 301–320.
M. E. El-Naggar, F. Al-Joufi, M. Anwar, M. F. Attia, and M. A. El-Bana (2019). Curcumin-loaded PLA-PEG copolymer nanoparticles for treatment of liver inflammation in streptozotocin-induced diabetic rats. Colloids Surf. B 177, 389–398.
R. Jagannathan, P. M. Abraham, and P. Poddar (2012). Temperature-dependent spectroscopic evidences of curcumin in aqueous medium: a mechanistic study of its solubility and stability. J. Phys. Chem. B 116 (50), 14533–14540.
R. K. Basniwal, H. S. Buttar, V. Jain, and N. Jain (2011). Curcumin nanoparticles: preparation, characterization, and antimicrobial study. J. Agric. Food Chem. 59 (5), 2056–2061.
M. Y. B. Hamzah, S. Hashim, and W. A. Wan Abd Rahman (2017). Gamma radiation-induced synthesis of nanocurcumin: characterization and cell viability test. Int. J. Polym. Mater. Polym. Biomater. 66 (18), 926–933.
Q. Nie, W. B. Tan, and Y. Zhang (2005). Synthesis and characterization of monodisperse chitosan nanoparticles with embedded quantum dots. Nanotechnology 17 (1), 140.
A. Kheiri, S. M. Jorf, A. Malihipour, H. Saremi, and M. Nikkhah (2017). Synthesis and characterization of chitosan nanoparticles and their effect on Fusarium head blight and oxidative activity in wheat. Int. J. Biol. Macromol. 102, 526–538.
M. N. Ali, T. M. Al-Saadi, and J. K. Al-Faragi (2021). Effect of Chitosan nanoparticles loaded oxytetracycline hydrochloride on health status of common carp (Cyprinuscarpio L.) infected with columnaris disease. J. Phys. 1879, 032075.
Z. Sayyar and H. Jafarizadeh-Malmiri (2020). Process intensification for curcumin nanodispersion preparation using subcritical water—optimization and characterization. Chem. Eng. Process. Process Intensif. 153, 107938.
G. Yang, Y. Wang, Z. Cui, B. Due, and G. Yin (2018). Expression and analysis of EPOR after radiation injury of salivary glands in rats. J. Odontol. 2 (101), 2.
M. Modasiya and V. Patel (2012). Studies on solubility of curcumin. Int. J. Pharm. Life Sci. 3 (3), 1490–1497.
P. Sankar, A. G. Telang, K. Ramya, K. Vijayakaran, M. Kesavan, and S. N. Sarkar (2014). Protective action of curcumin and nano-curcumin against arsenic-induced genotoxicity in rats in vivo. Mol. Biol. Rep. 41 (11), 7413–7422.
M. J. Laudenslager, J. D. Schiffman, and C. L. Schauer (2008). Carboxymethyl chitosan as a matrix material for platinum, gold, and silver nanoparticles. Biomacromolecules 9 (10), 2682–2685.
T. I. Jeon, S. G. Hwang, N. G. Park, Y. R. Jung, S. Im Shin, S. D. Choi, and D. K. Park (2003). Antioxidative effect of chitosan on chronic carbon tetrachloride induced hepatic injury in rats. Toxicology 187 (1), 67–73.
M.-C. Giroux, P. Hélie, P. Burns, and P. VaCHon (2015). Anesthetic and pathological changes following high doses of ketamine and xylazine in Sprague Dawley rats. Exp. Anim. 64 (3), 253–260.
I. Y. Abdelrahman, H. El-Kashef, and N. H. Hassan (2020). Anti-tumor effect of green tea extract, simvastatin and gamma radiation on solid tumor in mice. Arab J. Nucl. Sci. Appl. 53 (4), 39–52.
A. Baraka, S. Dickson, M. Gobara, G. S. El-Sayyad, M. Zorainy, M. I. Awaad, H. Hatem, M. M. Kotb, and A. Tawfic (2017). Synthesis of silver nanoparticles using natural pigments extracted from Alfalfa leaves and its use for antimicrobial activity. Chem. Pap. 71 (11), 2271–2281.
K. Franks, V. Kestens, A. Braun, G. Roebben, and T. P. Linsinger (2019). Non-equivalence of different evaluation algorithms to derive mean particle size from dynamic light scattering data. J. Nanopart. Res. 21 (9), 1–10.
R. Xu, C. Wu, and H. Xu (2007). Particle size and zeta potential of carbon black in liquid media. Carbon 45 (14), 2806–2809.
P. C. Soema, G.-J. Willems, W. Jiskoot, J.-P. Amorij, and G. F. Kersten (2015). Predicting the influence of liposomal lipid composition on liposome size, zeta potential and liposome-induced dendritic cell maturation using a design of experiments approach. Eur. J. Pharm. Biopharm. 94, 427–435.
S. Ravindran, M. Williams, R. Ward, and G. Gillies (2018). Understanding how the properties of whey protein stabilized emulsions depend on pH, ionic strength and calcium concentration, by mapping environmental conditions to zeta potential. Food Hydrocoll. 79, 572–578.
D. Meirow and D. Nugent (2001). The effects of radiotherapy and chemotherapy on female reproduction. Hum. Reprod. Update 7 (6), 535–543.
S.-Y. Yin, W.-C. Wei, F.-Y. Jian, and N.-S. Yang (2013). Therapeutic applications of herbal medicines for cancer patients. Evid. Based Complement. Altern. Med. 2013, 1.
N. M. D. Sandhiutami, W. Arozal, M. Louisa, D. Rahmat, and T. Mandy (2019). Comparative effect of curcumin and nanocurcumin on nephroprotection at cisplatin-induced rats. J. Pharm. Bioallied Sci. 11 (Suppl 4), S567.
J. Sharifi-Rad, C. Quispe, M. Butnariu, L. S. Rotariu, O. Sytar, S. Sestito, S. Rapposelli, M. Akram, M. Iqbal, and A. Krishna (2021). Chitosan nanoparticles as a promising tool in nanomedicine with particular emphasis on oncological treatment. Cancer Cell Int. 21 (1), 1–21.
C. Borek (2004). Antioxidants and radiation therapy. J. Nutr. 134 (11), 3207S-3209S.
R. T. Stramandinoli-Zanicotti, L. M. Sassi, J. L. Schussel, M. F. Torres, M. Funchal, G. H. Smaniotto, J. L. Dissenha, and A. L. Carvalho (2013). Effect of fractionated radiotherapy on the parotid gland: an experimental study in Brazilian minipigs. Int. Arch. Otorhinolaryngol. 17 (02), 163–167.
A. V. Shubin, I. V. Demidyuk, A. A. Komissarov, L. M. Rafieva, and S. V. Kostrov (2016). Cytoplasmic vacuolization in cell death and survival. Oncotarget 7 (34), 55863.
B. Short (2014). Exploring the LINC to nuclear envelope spacing. J. Cell Biol. 206 (2), 146.
Q. Shi and R. W. King (2005). Chromosome nondisjunction yields tetraploid rather than aneuploid cells in human cell lines. Nature 437 (7061), 1038–1042.
K. Taniguchi, A. Kokuryo, T. Imano, R. Minami, H. Nakagoshi, and T. Adachi-Yamada (2014). Isoform-specific functions of Mud/NuMA mediate binucleation of Drosophilamale accessory gland cells. BMC Dev. Biol. 14 (1), 1–18.
K. J. Jasmer, K. E. Gilman, K. Muñoz Forti, G. A. Weisman, and K. H. Limesand (2020). Radiation-induced salivary gland dysfunction: mechanisms, therapeutics and future directions. J. Clin. Med. 9 (12), 4095.
S. Cheng, V. Wu, D. Kwong, and M. Ying (2011). Assessment of post-radiotherapy salivary glands. Br. J. Radiol. 84 (1001), 393–402.
C. Winter, R. Keimel, M. Gugatschka, D. Kolb, G. Leitinger, and E. Roblegg (2021). Investigation of changes in saliva in radiotherapy-induced head neck cancer patients. Int. J. Environ. Res. Public Health 18 (4), 1629.
M. M. Curi, C. L. Cardoso, H. G. De Lima, L. P. Kowalski, and M. D. Martins (2016). Histopathologic and histomorphometric analysis of irradiation injury in bone and the surrounding soft tissues of the jaws. J. Oral Maxillofac. Surg. 74 (1), 190–199.
A. Magenta, S. Greco, C. Gaetano, and F. Martelli (2013). Oxidative stress and microRNAs in vascular diseases. Int. J. Mol. Sci. 14 (9), 17319–17346.
L. Huang and L. Zhang (2019). Neural stem cell therapies and hypoxic-ischemic brain injury. Prog. Neurobiol. 173, 1–17.
S. Hani, A. E. Abd Elmawla, S. Ahmed, and A. Emran (2018). Histological and immunohistochemical evaluation for the effect of pilocarpine and quercetin on gamma-irradiated parotid salivary glands. Egypt. J. Radiat. Sci. Appl. 31 (2), 177–184.
S. Shintani, A. Kiyota, M. Mihara, Y. Nakahara, N. Terakado, Y. Ueyama, and T. Matsumura (2000). Association of preoperative radiation effect with tumor angiogenesis and vascular endothelial growth factor in oral squamous cell carcinoma. Jpn. J. Cancer Res. 91 (10), 1051–1057.
H. Sies (1997). Oxidative stress: oxidants and antioxidants. Exp. Physiol. 82 (2), 291–295.
I. Brouet and H. Ohshima (1995). Curcumin, an anti-tumour promoter and anti-inflammatory agent, inhibits induction of nitric oxide synthase in activated macrophages. Biochem. Biophys. Res. Commun. 206 (2), 533–540.
M. Abd Elkodous, G. S. El-Sayyad, I. Y. Abdelrahman, H. S. El-Bastawisy, A. E. Mohamed, F. M. Mosallam, H. A. Nasser, M. Gobara, A. Baraka, M. A. Elsayed, and A. I. El-Batal (2019). Therapeutic and diagnostic potential of nanomaterials for enhanced biomedical applications. Colloids Surf. 180, 411–428.
H. Yavarpour-Bali, M. Ghasemi-Kasman, and M. Pirzadeh (2019). Curcumin-loaded nanoparticles: a novel therapeutic strategy in treatment of central nervous system disorders. Int. J. Nanomed. 14, 4449–4460.
Z. Delavarian, A. Pakfetrat, A. Ghazi, M. R. Jaafari, F. Homaei Shandiz, Z. Dalirsani, A. H. Mohammadpour, and H. R. Rahimi (2019). Oral administration of nanomicelle curcumin in the prevention of radiotherapy-induced mucositis in head and neck cancers. Spec. Care Dent. 39 (2), 166–172.
Z. Liu, P. Huang, S. Law, H. Tian, W. Leung, and C. Xu (2018). Preventive Effect of Curcumin Against Chemotherapy-Induced Side-Effects. Front Pharmacol. https://doi.org/10.3389/fphar.2018.01374.
D. G. Binion, M. F. Otterson, and P. Rafiee (2008). Curcumin inhibits VEGF-mediated angiogenesis in human intestinal microvascular endothelial cells through COX-2 and MAPK inhibition. Gut 57 (11), 1509–1517.
J. Jhaveri, Z. Raichura, T. Khan, M. Momin, and A. Omri (2021). Chitosan nanoparticles-insight into properties, functionalization and applications in drug delivery and theranostics. Molecules 26 (2), 272.
T. L. Yang and T. H. Young (2009). The specificity of chitosan in promoting branching morphogenesis of progenitor salivary tissue. Biochem. Biophys. Res. Commun. 381 (4), 466–470.
Z. Huang, L. Dong, J. Chen, F. Gao, Z. Zhang, J. Chen, and J. Zhang (2012). Low-molecular weight chitosan/vascular endothelial growth factor short hairpin RNA for the treatment of hepatocellular carcinoma. Life Sci. 91 (23–24), 1207–1215.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors stated and declare that no funders for this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors stated and declare that No conflict or competing of interests.
Consent to Participate
No experimental investigation was performed on individuals within this research study.
Consent for Publication
All the co-authors are agreed for the research study publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Meabed, O.M., Shamaa, A., Abdelrahman, I.Y. et al. The Effect of Nano-chitosan and Nano-curcumin on Radiated Parotid Glands of Albino Rats: Comparative Study. J Clust Sci 34, 977–989 (2023). https://doi.org/10.1007/s10876-022-02281-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-022-02281-y