Abstract
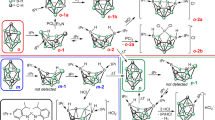
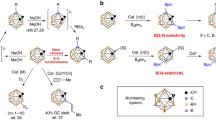
We have studied the addition of reaction between iminoborane HBNH with the BnNn cages (n = 12, 16, 28 and 36) for the chemoselectivity (BN bond cleavage and expansion ring vs [2 + 2] cycloaddition) and regioselectivity (square–hexagon junctions vs hexagon–hexagon) of the reaction. Based on our results, the iminoborane molecule can either selectively break a B–N bond of the BN cages, expanding the square ring of the BN cage to a larger one at the surface, or undergo a [2 + 2]-cycloaddition on the BNNT surface. These reactions exhibit a depending on the reactive site of the cages. The square–hexagon B–N bond of the cages prefer bond-cleavage-ring-expansion processes, while hexagon–hexagon B–N bonds follow [2 + 2] cycloaddition reaction. Overall, all reactions are is exothermic while bond-cleavage-ring-expansion processes are a bit more favorable than [2 + 2] cycloadditions with smaller barrier heights. While complexes of iminoborane with hexagon–hexagon bonds at the middle of the larger cages resemble [2 + 2]-cycloaddition, BN bond cleavage also occurred and HBNH acts as a bridge at the top of the decagon. The larger values of HOMO–LUMO gaps for the most stable configurations also indicate kinetically preference the BN bond cleavage and ring expansion processes than the [2 + 2]-cycloaddition reactions.






Similar content being viewed by others
References
F. Zhang, P. Maksyutenko, R. I. Kaiser, A. M. Mebel, A. Gregušová, S. A. Perera, and R. J. Bartlett (2010). J. Phys. Chem. A 114, 12148.
Y. Kawashima, K. Kawaguchi, and E. Hirota (1987). J. Chem. Phys. 87, 6331.
E. R. Lory and R. F. Porter (1973). J. Am. Chem. Soc. 95, 1766.
A. Meller Topics in Current Chemistry (Springer, Berlin, 1972).
P. Paetzold, H. J. Emeléus, and A. G. Sharpe, Advances in Inorganic Chemistry, vol. 31, Chap. Iminoboranes (Academic Press, Orlando, FL, 1987), pp. 123-170.
J. D. Dill, P. V. R. Schleyer, and J. A. Pople (1975). J. Am. Chem. Soc. 97, 3402.
P. Botschwina (1978). Chem. Phys. 28, 231.
D. V. Lanzisera and L. Andrews (1997). J. Phys. Chem. A 101, 824.
V. M. Rosas-Garcia (2011). Comput. Theor. Chem. 967, 160.
N. C. Baird and R. K. Datta (1972). Inorg. Chem. 11, 17.
T. M. Gilbert (2003). Organometallics 22, 2298.
T. M. Gilbert and S. M. Bachrach (2007). Organometallics 26, 2672.
T. M. Gilbert (1998). Organometallics 17, 5513.
T. M. Gilbert (2000). Organometallics 19, 1160.
T. M. Gilbert and B. D. Gailbreath (2001). Organometallics 20, 4727.
P. Paetzold (1991). Pure Appl. Chem. 63, 345.
D. R. Armstrong and D. T. Clark (1972). Theor. Chem. Acc. 24, 307.
E. V. Steuber, G. Elter, M. Noltemeyer, H.-G. Schmidt, and A. Meller (2000). Organometallics 19, 5083.
R. Sundaram, S. Scheiner, A. K. Roy, and T. Kar (2015). J. Phys. Chem. C 119, 3253.
F. Jensen and H. Toflund (1993). Chem. Phys. Lett. 201, 89.
H. Y. Zhu, T. G. Schmaltz, and D. J. Klein (1997). Int. J. Quantum Chem. 63, 393.
G. Seifert, R. W. Fowler, D. Mitchell, D. Porezag, and T. Frauenheim (1997). Chem. Phys. Lett. 268, 352.
R. T. Paine and C. K. Narula (1990). Chem. Rev. 90, 73.
T. Oku, T. Hirano, M. Kuno, T. Kusunose, K. Niihare, and K. Suganuma (2000). Mater. Sci. Eng. B 74, 206.
T. Oku, M. Kuno, H. Kitahara, and I. Nartia (2001). Int. J. Inorg. Mater. 3, 597.
S. S. Alexandre, M. S. C. Mazzoni, and H. Chacham (1999). Appl. Phys. Lett. 75, 61.
V. V. Pokropivny, V. V. Skorokhod, G. S. Oleinik, A. V. Kurdyumov, T. S. Bartnits-kaya, A. V. Pokropivny, A. G. Sisonyuk, and D. M. Sheichenko (2000). J. Solid State Chem. 154, 214.
D. Golberg, Y. Bando, O. Stephan, and K. Kurashima (1998). Appl. Phys. Lett. 73, 2441.
J. M. L. Martin, J. El-Yazal, J. P. Francois, and R. Gijbels (1995). Chem. Phys. Lett. 232, 289.
J. M. L. Martin, J. El-Yazal, and J. P. Francois (1996). Chem. Phys. Lett. 248, 95.
D. L. Strout (2000). J. Phys. Chem. A 104, 3364.
D. L. Strout (2001). J. Phys. Chem. A 105, 261.
S. Osuna and K. N. Houk (2009). Chem. Eur. J. 15, 13219.
Y. Zhao and D. G. Truhlar (2008). Theor. Chem. Account. 120, 215.
M. Anafcheh and R. Ghafouri (2014). Comput. Theor. Chem. 1034, 32.
M. Anafcheh (2018). Mol. Phys. 116, 179.
M. W. Schmidt, K. K. Baldridge, J. A. Boatz, S. T. Elbert, M. S. Gordon, J. H. Jensen, S. Koseki, N. Matsunaga, K. A. Nguyen, S. J. Su, T. L. Windus, M. Dupuis, and J. A. Montgomery (1993). J. Comput. Chem. 14, 1347.
M. S. Gordon and M. W. Schmidt, in C. E. Dykstra, G. Frenking, K. S. Kim, and G. E. Scuseria (eds.), Theory and Applications of Computational Chemistry: The First Forty Years (Elsevier, Amsterdam, 2005).
V. Barone, A. Koller, and G. E. Scuseria (2006). J. Phys. Chem. A 110, 10844.
S. F. Boys and F. Bernardi (1970). Mol. Phys. 19, 553.
J. F. Gonthier, S. N. Steinmann, L. Roch, A. Ruggi, N. Luisier, K. Severin, and C. Corminboeuf (2012). Chem. Commun. 48, 9239.
R. D. Johnson, in NIST Computational Chemistry Comparison and Benchmark Database III, (NIST, Washington, DC, 2006).
Acknowledgements
We gratefully acknowledge for the financial support from the Research Council of Alzahra University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Anafcheh, M., Ghazi Mir Saeed, S. & Zahedi, M. [2 + 2] Cycloaddition and Bond Cleavage of Boron Nitride Cages with Iminoborane: A DFT Study. J Clust Sci 33, 29–35 (2022). https://doi.org/10.1007/s10876-020-01933-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-020-01933-1