Abstract
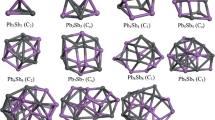
The structural, relative stable and electronic properties of PbnSnn (n = 2–12) alloy clusters were systematically studied using density functional theory. The isomers of PbnSnn alloy clusters were generated and determined by ab initio molecular dynamics. By comparing the calculated parameters of Pb2 dimer and Sn2 dimers with the parameters from experiments, our calculations are reasonable. With the lowest-energy structures for PbnSnn clusters, the average binding energies, fragmentation energies, second- order energy differences, vertical ionization potentials, vertical electron affinities, HOMO–LUMO gaps, and density of states were calculated and analyzed. The results indicate that the Sn atoms have a tendency to bond together, the average binding energies tend to be stable up to n = 8, Pb8Sn8 cluster is a good candidate to calculate the molecular interaction energy parameter in Wilson equation, the clusters become less chemical stable and show an insulator-to-metallic transition, 3, 6, 8 and 11 are magic numbers of PbnSnn (n = 2–12) clusters, the charges always transfer from Sn atoms to Pb atoms in PbnSnn clusters except for Pb10Sn10 cluster, and density of states of PbnSnn clusters becoming continuous and shifting toward negative with the increasing size n.









Similar content being viewed by others
References
C. T. Campbell (2013). The Energetics of Supported Metal Nanoparticles: Relationships to Sintering Rates and Catalytic Activity. Acc. Chem. Res. 46, 1712.
H. Zhang, M. Jin, Y. Xiong, B. Lim, and Y. Xia (2013). Shape-Controlled Synthesis of Pd Nanocrystals and Their Catalytic Applications. Acc. Chem. Res. 46, 1783.
D. Cortes-Arriagada, M. P. Oyarzun, L. Sanhueza, and A. Toro-Labbe (2015). Binding of Trivalent Arsenic onto the Tetrahedral Au20 and Au19Pt Clusters: Implications in Adsorption and Sensing. J. Phys. Chem. A 119, 6909.
S. Hirabayashi and M. Ichihashi (2015). NO Decomposition Activated by Preadsorption of O2 onto Copper Cluster Anions. J. Phys. Chem. C 119, 10850.
Z. Ben-Xia, D. Dong, W. Ling, and Y. Ji-Xian (2014). Density functional study on the structural, electronic, and magnetic properties of 3d transition-metal-doped Au5 clusters. J. Phys. Chem. A 118, 4005.
F. Aguilera-Granja, M. B. Torres, A. Vega, and L. C. Balbas (2012). Structural, electronic, and magnetic properties Of ConCum nanoalloys (m + n = 12) from first principles calculations. J. Phys. Chem. A 116, 9353.
P. Chen, Y. Li, J. Ma, J. Huang, C. Chen, and H. Chang (2016). Size-tunable copper nanocluster aggregates and their application in hydrogen sulfide sensing on paper-based devices. Sci. Rep. 6, 24882.
J. N. Anker, W. P. Hall, O. Lyandres, N. C. Shah, J. Zhao, and R. P. Van Duyne (2008). Biosensing with plasmonic nanosensors. Nat. Mater. 7, 442.
W. Ma and F. Chen (2012). Optical and electronic properties of Cu doped Ag clusters. J. Alloys Compd. 541, 79.
S. J. Oldenburg, J. B. Jackson, S. L. Westcott, and N. J. Halas (1999). Infrared extinction properties of gold nanoshells. Appl. Phys. Lett. 75, 2897.
M. Zhou, C. Zeng, Y. Chen, S. Zhao, M. Y. Sfeir, M. Zhu, and R. Jin (2016). Evolution from the plasmon to exciton state in ligand-protected atomically precise gold nanoparticles. Nat. Commun. 7, 13240.
R. Jin, C. Zeng, M. Zhou, and Y. Chen (2016). Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities. Chem. Rev. 116, 10346.
O. Fenwick, E. Coutino-Gonzalez, D. Grandjean, W. Baekelant, F. Richard, S. Bonacchi, D. De Vos, P. Lievens, M. Roeffaers, J. Hofkens, and P. Samori (2016). Tuning the energetics and tailoring the optical properties of silver clusters confined in zeolites. Nat. Mater. 15, 1017.
G. Seifert (2004). Nanomaterials: Nanocluster magic. Nat. Mater. 3, 77.
X. Yang, X. Chen, C. Zhang, X. Xie, B. Yang, B. Xu, D. Liu, and H. Yang (2016). Prediction of vapor–liquid equilibria for the Pb–X (X = Ag, Cu and Sn) systems in vacuum distillation using ab initio methods and Wilson equation. Fluid Phase Equilib. 417, 25.
A. K. Sum and S. I. Sandler (1999). Use of ab initio methods to make phase equilibria predictions using activity coefficient models. Fluid Phase Equilib. 158–160, 375.
S. Osmekhin, M. Tchaplyguine, M. H. Mikkelä, M. Huttula, T. Andersson, O. Björneholm, and S. Aksela (2010). Size-dependent transformation of energy structure in free tin clusters studied by photoelectron spectroscopy. Phys. Rev. A 81, 023203.
M. Tchaplyguine, G. Öhrwall, T. Andersson, S. Svensson, O. Björneholm, M. Huttula, M. Mikkelä, S. Urpelainen, S. Osmekhin, A. Caló, S. Aksela, and H. Aksela (2014). Size-dependent evolution of electronic structure in neutral Pb clusters—As seen by synchrotron-based X-ray photoelectron spectroscopy. J. Electron Spectrosc. 195, 55.
J. Bahn, P. Oelßner, M. Köther, C. Braun, V. Senz, S. Palutke, M. Martins, E. Rühl, G. Ganteför, T. Möller, B. von Issendorff, D. Bauer, J. Tiggesbäumker, and K. H. Meiwes-Broer (2012). Pb 4f photoelectron spectroscopy on mass-selected anionic lead clusters at FLASH. New J. Phys. 14, 075008.
B. Wang, J. Zhao, X. Chen, D. Shi, and G. Wang (2005). Atomic structures and covalent-to-metallic transition of lead clusters Pbn (n = 2–22). Phys. Rev. A 71, 033201.
B. Assadollahzadeh, S. Schäfer, and P. Schwerdtfeger (2010). Electronic properties for small tin clusters Snn (n ≤ 20) from density functional theory and the convergence toward the solid state. J. Comput. Chem. 31, 929.
C. Majumder, V. Kumar, H. Mizuseki, and Y. Kawazoe (2001). Small clusters of tin: Atomic structures, energetics, and fragmentation behavior. Phys. Rev. B 64, 233405.
C. Majumder, V. Kumar, H. Mizuseki, and Y. Kawazoe (2002). Ionization potentials of small tin clusters: first principles calculations. Chem. Phys. Lett. 356, 36.
C. Majumder, V. Kumar, H. Mizuseki, and Y. Kawazoe (2005). Atomic and electronic structures of neutral and cation Snn (n = 2–20) clusters: A comparative theoretical study with different exchange-correlation functionals. Phys. Rev. B 71, 035401.
T. Bachels and R. Schäfer (1999). Formation enthalpies of Sn clusters: a calorimetric investigation. Chem. Phy. Lett. 300, 177.
S. Yahachi, Y. Kenzi, M. Kazuhiro, and N. Tamotsu (1982). Formation and Ionization Potentials of Lead Clusters. Jpn. J. Appl. Phys. 21, L396.
C. Rajesh and C. Majumder (2007). Atomic and electronic structures of neutral and charged Pbn clusters (n = 2–15): theoretical investigation based on density functional theory. J. Chem. Phys. 126, 244704.
X. Li, W. Lu, C. Wang, and K. M. Ho (2010). Structures of Pbn (n = 21–30) clusters from first-principles calculations. J. Phys. Condens. Matter 22, 465501.
E. M. Sosa-Hernández, J. M. Montejano-Carrizales, and P. G. Alvarado-Leyva (2015). Stability and magnetic behavior of small CoxSny (x + y ≤ 5) atomic clusters. J. Alloys Compd. 632, 772.
P. N. Samanta and K. K. Das (2012). Electronic structure, bonding, and properties of SnmGen (m + n ⩽ 5) clusters: A DFT study. Comput. Theor. Chem. 980, 123.
E. M. Sosa-Hernández, J. M. Montejano-Carrizales, and P. G. Alvarado Leyva (2015). Geometrical shapes, stabilities and electronic behavior of small FexSny (x + y ≤ 5) atomic clusters. Eur. Phys. J. D 69, 212.
T. B. Tai, N. M. Tam, and M. T. Nguyen (2011). Evolution of structures and stabilities of zinc-doped tin clusters SnnZn, n = 1–12. Three-dimensional aromaticity of the magic clusters Sn10Zn and Sn12Zn. Chem. Phys. 388, 1.
L. O. Paz-Borbon, A. Hellman, J. M. Thomas, and H. Gronbeck (2013). Efficient hydrogenation over single-site bimetallic RuSn clusters. Phys. Chem. Chem. Phys. 15, 9694.
J. J. Melko, U. Werner, R. Mitric, V. Bonacic-Koutecky, and A. W. Castleman Jr. (2011). Electronic structure similarities in PbxSb −y and SnxBi −y clusters. J. Phys. Chem. A 115, 10276.
X. Xing, Z. Tian, H. Liu, and Z. Tang (2003). Magic bimetallic cluster anions of M/Pb (M = Au, Ag and Cu) observed and analyzed by laser ablation and time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 17, 1411.
J. J. Melko, S. V. Ong, U. Gupta, J. U. Reveles, J. D’Emidio, S. N. Khanna, and A. W. Castleman (2010). Anion Photoelectron Spectroscopy and First-Principles Study of PbxIny Clusters. J. Phys. Chem. C 114, 20907.
C. Rajesh and C. Majumder (2008). Structure and electronic properties of PbnM (M = C, Al, In, Mg, Sr, Ba, and Pb; n = 8, 10, 12, and 14) clusters: theoretical investigations based on first principles calculations. J. Chem. Phys. 128, 024308.
S. Barman, C. Rajesh, G. P. Das, and C. Majumder (2009). Structural and electronic properties of Snn−1Pb and Pbn−1Sn clusters: a theoretical investigation through first principles calculations. Eur. Phys. J. D 55, 613.
S. Orel and R. Fournier (2013). Density functional theory and global optimization study of SnmPbn clusters (7 ⩽ m + n ⩽ 12, 0 ⩽ m/(m + n) ⩽ 1). J. Chem. Phys. 138, 064306.
X. Huang, Y. Su, L. Sai, J. Zhao, and V. Kumar (2015). Low-Energy Structures of Binary Pt–Sn Clusters from Global Search Using Genetic Algorithm and Density Functional Theory. J. Clust. Sci. 26, 389.
V. E. Bondybey, M. Heaven, and T. A. Miller (1983). Laser vaporization of tin: Spectra and ground state molecular parameters of Sn2. J. Chem. Phys. 78, 3593.
S. Yoshida and K. Fuke (1999). Photoionization studies of germanium and tin clusters in the energy region of 5.0–8.8 eV: Ionization potentials for Gen (n = 2–57) and Snn (n = 2–41). J. Chem. Phys. 111, 3880.
C. Rajesh, C. Majumder, M. G. R. Rajan, and S. K. Kulshreshtha (2005). Isomers of small Pbn clusters (n = 2–15): Geometric and electronic structures based onab initiomolecular dynamics simulations. Phys. Rev. B 72, 235411.
M. E. Eberhart, R. C. O’Handley, and K. H. Johnson (1984). Molecular-orbital models of structural phase transformations in crystalline and amorphous cobalt alloys. Phys. Rev. B 29, 1097.
Y. Bai, H. Cheng, H. Sun, N. Xu, and K. Deng (2011). Structures, stabilities and electronic properties of FePbn (n = 1–14) clusters: Density-functional theory investigations. Physica B 406, 3781.
J. Wen, J. Zhang, G. Chen, X. Zhang, and Z. Wen (2016). Structure, stability and magnetic properties of (NiAl)n(n ≤ 6) clusters. J. Phys. Chem. Solids 96–97, 68.
F. Suo, Y. Zhang, and S. Huang (2017). Theoretical Investigation of Electronic Properties of Undoped and Ag-Doped (CdTe)16×N Multi-cage Nanochains. J. Clust. Sci. 28, 1393.
K. Li, C. Yang, M. Wang, and X. Ma (2017). Adsorption and Dissociation of H2 on Cluster Al6N. J. Clust. Sci. 28, 1335.
Y. Jin, Y. Tian, X. Kuang, C. Zhang, C. Lu, J. Wang, J. Lv, L. Ding, and M. Ju (2015). Ab Initio Search for Global Minimum Structures of Pure and Boron Doped Silver Clusters. J. Phys. Chem. A 119, 6738.
X. X. Xia, A. Hermann, X. Y. Kuang, Y. Y. Jin, C. Lu, and X. D. Xing (2016). Study of the Structural and Electronic Properties of Neutral and Charged Niobium-Doped Silicon Clusters: Niobium Encapsulated in Silicon Cages. J. Phys. Chem. C 120, 677.
G. Li, J. Wang, X. Chen, Z. Zhou, H. Yang, B. Yang, B. Xu, and D. Liu (2017). Bimetallic PbnCun (n = 2–14) clusters were investigated by density functional theory. Comput. Theor. Chem. 1106, 21.
Y. Jin, G. Maroulis, X. Kuang, L. Ding, C. Lu, J. Wang, J. Lv, C. Zhang, and M. Ju (2015). Geometries, stabilities and fragmental channels of neutral and charged sulfur clusters: S Qn (n = 3–20, Q = 0, ±1). Phys. Chem. Chem. Phys. 17, 13590.
X. Xing, A. Hermann, X. Kuang, M. Ju, C. Lu, Y. Jin, X. Xia, and G. Maroulis (2016). Insights into the geometries, electronic and magnetic properties of neutral and charged palladium clusters. Sci. Rep. 6, 19656.
W. G. Sun, J. J. Wang, C. Lu, X. X. Xia, X. Y. Kuang, and A. Hermann (2017). Evolution of the Structural and Electronic Properties of Medium-Sized Sodium Clusters: A Honeycomb-Like Na20 Cluster. Inorg. Chem. 56, 1241–1248.
Acknowledgements
This work was supported by the Regional Foundation of the National Natural Science Foundation of China (51664032), the Foundation of the State Key Laboratory of Complex Nonferrous Metal Resources Clear Utilization (CNMRCUTS1503), the Joint Foundation of the National Natural Science Foundation of China–Yunnan province (U1502271), the Cultivating Plan Program for the Leader in Science and Technology of Yunnan Province (2014HA003), the Program for Nonferrous Metals Vacuum Metallurgy Innovation Team of Ministry of Science and Technology (2014RA4018) and the National Key Research and Development Program of China (2016YFC0400404).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Gf., Zhou, Zq., Chen, Xm. et al. Structural, Relative Stable, and Electronic Properties of PbnSnn (n = 2–12) Clusters were Investigated Using Density Functional Theory. J Clust Sci 28, 2503–2516 (2017). https://doi.org/10.1007/s10876-017-1242-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-017-1242-9