Abstract
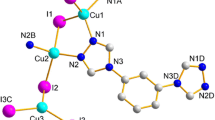
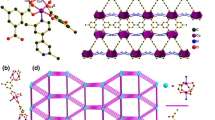
The CuX salts (X = Br, I) react with 1,4-bis(cyclohexylthio)butane, L2, in a 1:1 ratio to form the corresponding isostructural and weakly luminescent 1D coordination polymers [(Cu2X2)(μ-L2)2] n (X = Br, 4; X = I, 3) as determined by X-ray crystallography. The previously reported reaction of CuI with 1,4-bis(phenylthio)butane, L1, in a 2:1 metal-to-ligand ratio provides a 2D polymer [(Cu4I4)(μ-L1)2] n , 1 (Knorr et al., Dalton Trans 38:948–955, 2009), where the Cu4I4 unit exhibits the common cubane structure and an intense luminescence centered at 555 and 565 nm respectively at 298 and 77 K. When CuI reacts with L2 in a 2:1 metal-to-ligand ratio, a new material 2 is formed but no X-ray structure was obtained. The intense and characteristic luminescence of polymer 2 is strongly indicative of the formation of the cubane Cu4I4 unit. The new materials have been characterized by solid-state UV–Vis rasing-angle transmittance spectroscopy, luminescence spectroscopy and emission lifetime measurements.
Graphical Abstract







Similar content being viewed by others
References
I.-H. Park, H. J. Kim, and S. S. Lee (2012). Cryst. Eng. Comm. 14, 4589.
I.-H. Park and S. S. Lee (2011). Cryst. Eng. Comm. 13, 6520.
J. Zhang, Y.-S. Xue, Y.-Z. Li, H.-B. Du, and X.-Z. You (2011). Cryst. Eng. Comm. 13, 2578.
Y. Jin, H. J. Kim, H. Y. Lee, S. Y. Lee, W. J. Shim, S. H. Hong, and S. S. Lee (2010). Inorg. Chem. 49, 10241.
E.-J. Kang, S. Y. Lee, H. Lee, and S. S. Lee (2010). Inorg. Chem. 49, 7510.
C. Xie, L. Zhou, W. Feng, J. Wang, and W. Chen (2009). J. Mol. Struct. 921, 132.
J. Y. Lee, S. Y. Lee, W. Sim, K.-M. Park, J. Kim, and S. S. Lee (2008). J. Am. Chem. Soc. 130, 6902.
T. H. Kim, G. Park, Y. W. Shin, K.-M. Park, M. Y. Choi, and J. Kim (2008). J. Bull. Korean Chem. Soc. 29, 499.
T. H. Kim, Y. W. Shin, J. H. Jung, J. S. Kim, and J. Kim (2008). Angew. Chem. Int. Ed. 47, 685.
T. H. Kim, Y. W. Shin, S. S. Lee, and J. Kim (2007). Inorg. Chem. Commun. 10, 11.
T. H. Kim, K. Y. Lee, Y. W. Shin, S.-T. Moon, K.-M. Park, J. S. Kim, Y. Kang, S. S. Lee, and J. Kim (2005). Inorg. Chem. Commun. 8, 27.
P. D. Harvey and M. Knorr (2010). Macromol. Rapid Commun. 31, 808.
H.-B. Zhu (2010). Acta Cryst. E 66, m41.
H. N. Peindy, F. Guyon, A. Khatyr, M. Knorr, V. H. Gessner, and C. Strohmann (2009). Anorg. Allg. Chem. 635, 2099–2105.
S.-Y. Lee, S. Park, and S. S. Lee (2009). Inorg. Chem. 48, 11335.
M. Jo, J. Seo, L. F. Lindoy, and S. S. Lee (2009). Dalton Trans. 31, 6096.
J. Kim, M. R. Song, S. Y. Lee, J. Y. Lee, and S. S. Lee (2008). Eur. J. Inorg. Chem. 22, 3532.
Y.-C. Yang, S.-T. Lin, and W.-S. Chen (2008). J. Chem. Res. 2008, 280.
W.-J. Shi, C.-X. Ruan, Z. Li, M. Li, and D. Li (2008). Cryst. Eng. Comm. 10, 778.
P. R. Martinez-Alanis, V. M. Ugalde-Saldivar, and I. Castillo (2011). Eur. J. Inorg. Chem., 212.
E. W. Ainscough, A. M. Brodie, A. Derwahl, G. H. Freeman, and C. A. Otter (2004). Polyhedron 26, 5398.
M. Heller and W. S. Sheldrick (2004). Z. Anorg. Allg. Chem. 630, 1869.
M. Heller and W. S. Sheldrick (2003). Z. Anorg. Allg. Chem. 629, 1589.
H. W. Yim, D. Rabinovich, K. C. Lam, K. Chung, J. A. Golen, and L. A. Rheingold (2003). Acta Cryst. Sec. E 59, m556.
B. Kure, S. Ogo, D. Inoki, H. Nakai, K. Isobe, and S. Fukuzumi (2005). J. Am. Chem. Soc. 127, 14366.
R. D. Adams, M. Huang, and S. Johnson (1998). Polyhedron 17, 2775.
Y. Suenaga, M. Maekawa, T. Kuroda-Sowa, M. Munakata, H. Morimoto, N. Hiyama, and S. Kitagawa (1997). Anal. Sci. 13, 1047.
L. I. Victoriano, M. T. Garland, and A. Vega (1997). Inorg. Chem. 36, 688.
M. Munakata, L. P. Wu, T. Kuroda-Sowa, M. Maekawa, Y. Suenaga, and S. Nakagawa (1996). J. Chem. Soc. Dalton Trans. 25, 1525.
L. I. Victoriano and B. H. Cortes (1995). J. Coord. Chem. 36, 159.
D. Mentzafos, A. Terzis, P. Karagiannidis, and P. Aslanidis (1989). Acta Cryst. Sec. C45, 54.
B. Noren and A. Oskarsson (1987). Acta Chem. Scand. A41, 12.
M. A. Tsiaggali, E. G. Andreadou, A. G. Hatzidimitriou, A. A. Pantazaki, and P. Aslanidis (2013). J. Inorg. Biochem. 121, 121.
H. N. Peindy, F. Guyon, A. Khatyr, M. Knorr, and C. Strohmann (2007). Eur. J. Inorg. Chem., 1823.
M. Knorr, F. Guyon, A. Khatyr, C. Strohmann, M. Allain, S. M. Aly, A. Lapprand, D. Fortin, and P. D. Harvey (2012). Inorg. Chem. 51, 9917.
M. Knorr, F. Guyon, M. M. Kubicki, Y. Rousselin, S. M. Aly, and P. D. Harvey (2011). New J. Chem. 35, 1184.
M. Knorr, A. Pam, A. Khatyr, C. Strohmann, M. M. Kubicki, Y. Rousselin, S. M. Aly, D. Fortin, and P. D. Harvey (2010). Inorg. Chem. 49, 5834.
M. Knorr, F. Guyon, A. Khatyr, C. Däschlein, C. Strohmann, S. M. Aly, A. S. Abd-El-Aziz, D. Fortin, and P. D. Harvey (2009). Dalton Trans. 38, 948.
M. Knorr, F. Guyon, A. Khatyr, M. Allain, S. M. Aly, A. Lapprand, D. Fortin, and P. D. Harvey (2010). J. Inorg. Organomet. Polym. Mat. 20, 534.
A. Lapprand, A. Bonnot, M. Knorr, Y. Rouselin, M. M. Kubicki, D. Fortin, and P. D. Harvey (2013). Chem. Commun. 49, 8848.
E. Anklam (1987). Synthesis 92, 841.
E. Anklam, K. D. Asmus, and H. Mohan (1990). J. Phys. Org. Chem. 3, 17.
Q. Wang, X.-Y. Li, G. K. S. Prakash, G. A. Olah, D. P. Loker, and K. B. Loker (2001). ARKIVOC 116, 1649.
P. C. Healy, J. D. Kildea, B. W. Skelton, and A. H. White (1989). Aust. J. Chem. 42, 79.
G. M. Sheldrick (2008). Acta Cryst. A64, 112.
H. N. Peindy, F. Guyon, M. Knorr, and C. Strohmann (2005). Z. Anorg. Allg. Chem. 631, 2397.
X.-H. Bu, W. Chen, W.-F. Hou, M. Du, R.-H. Zhang, and F. Brisse (2002). Inorg. Chem. 41, 3477.
W. Lu, Z.-M. Yan, J. Dai, Y. Zhang, Q.-Y.; Zhu, D.-X. Jia, and W.-J. Guo (2005). Eur. J. Inorg. Chem., 2339.
S. Toyota, Y. Matsuda, S. Nagaoka, M. Oki, and H. Akashi (1996). Bull. Chem. Soc. Jpn. 69, 3115.
C. Bellitto, F. Bigoli, P. Deplano, M. L. Mercuri, M. A. Pellinghelli, G. Staulo, and E. F. Trogu (1994). Inorg. Chem. 33, 3005.
C. R. Lucas, W. Liang, D. O. Miller, and J. N. Bridson (1997). Inorg. Chem. 36, 4508.
A. Hameau, F. Guyon, A. Khatyr, M. Knorr, and C. Strohmann (2012). Inorg. Chim. Acta 388, 60.
F. R. Knight, A. L. Fuller, A. M. Z. Slawin, and J. D. Woollins (2009). Dalton Trans. 38, 8476.
M. Vitale, C. K. Ryu, W. E. Palke, and P. C. Ford (1994). Inorg. Chem. 33, 561.
F. De Angelis, S. Fantacci, A. Sgamellotti, E. Cariati, R. Ugo, and P. C. Ford (2006). Inorg. Chem. 45, 10576.
A. Vega and J. Y. Saillard (2004). Inorg. Chem. 43, 4012.
S. Perruchas, C. Tard, X. F. Le Goff, A. Fargues, A. Garcia, S. Khalal, J. Y. Saillard, T. Gacoin, and J. P. Boilot (2011). Inorg. Chem. 50, 10682.
E. Jalilian, R.-Z. Liao, F. Himo, and S. Lidin (2011). Mat. Res. Bull. 46, 1192.
Acknowledgments
This research was supported by the CNRS, the Natural Sciences and Engineering Research Council of Canada (NSERC), le Fonds Québécois de la Recherche sur la Nature et les Technologies (FQRNT), and the Centre d’Etudes des Matériaux Optiques et Photoniques de l’Université de Sherbrooke (CEMOPUS).
Author information
Authors and Affiliations
Corresponding authors
Additional information
This paper is intended to the special issue of the 25th anniversary of the Journal of Cluster Science.
Rights and permissions
About this article
Cite this article
Bonnot, A., Strohmann, C., Knorr, M. et al. Metal-to-Ligand Ratio Effect on the Size of Copper Iodide and Copper Bromide Clusters in 1,4-Bis(cyclohexylthio)butane-Spanned Coordination Polymers. J Clust Sci 25, 261–275 (2014). https://doi.org/10.1007/s10876-013-0637-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-013-0637-5