Abstract
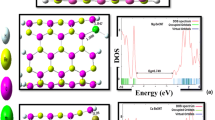
Adsorption of chlorine on the external surface of Mg15O15 nanotube was studied by using density functional calculations in terms of adsorption energy, energy gap changes, charge transfer, structural deformation, etc. It was found that the electronic properties of the Mg15O15 nanotube are very high sensitive to the presence of Cl2 and the nanotube can significantly detect Cl2 molecules. Also, the Cl2 molecules have not a strong interaction with the nanotube to hinder the recovery of the nanotube. Based on calculated results, the Mg15O15 nanotube can be potential sensor for Cl2 molecules detection.






Similar content being viewed by others
References
S. Ijima and T. Ichihashi (1993). Nature 6430, 603–605.
V. Derycke, R. Martel, J. Appenzeller, and Ph Avouris (2002). Appl. Phys. Lett. 80, 2773.
C. Liu, Y. Y. Fan, M. Liu, H. T. Cong, H. M. Cheng, and M. S. Dressel-haus (1999). Science 286, 1127.
B. Zurek and J. Autschbach (2004). J. Am. Chem. Soc. 126, 13079.
A. Nojeh, G. W. Lakatos, S. Peng, K. Cho, and R. F. W. Pease (2003). Nano Lett. 3, 1187.
J. P. Lu and J. Han (1998). Int. J. High Speed Electron. Syst. 9, 101.
Y. Zhen, H. W. C. Postma, L. Balents, and C. Dekker (1999). Nature 402, 273.
H. Gao, Y. Kong, D. Cui, and C. S. Ozkan (2003). Nano Lett. 3, 471.
M. T. Baei, A. R. Soltani, A. V. Moradi, and M. Moghimi (2011). Monatsh Chem. 142, 573.
M. T. Baei, A. Ahmadi, and M. Moghimi (2012). J. Mol. Model. 18, 4477.
J. Beheshtian, M. T. Baei, Z. Bagheri, and A. Ahmadi (2012). Struct. Chem. doi:10.1007/s11224-012-0021-3.
M. T. Baei, A. Ahmadi, and Z. Bagheri (2012). Chin. Chem. Lett. 23, 965.
J. Zhan, Y. Bando, J. Hu, and D. Golberg (2004). Inorg. Chem. 43, 2462.
J. Beheshtian, M. Kamfiroozi, Z. Bagheri, and A. Ahmadi (2011). Phys. E 44, 546.
Q. Shen, L. Li, J. Li, H. Tian, and Z. Hao (2009). J. Hazard. Mater. 163, 1332.
G. Bilalbegovic′ (2004). Phys. Rev. B 70, 045407.
G. Xiao, R. Singh, A. Chaffee, and P. Webley (2011). Int. J. Green House Gas Control 5, 634.
B. C. Gates Catalytic Chemistry (Wiley, Chichester, 1992), p. 393.
M. I. McCarthy and A. C. Hess (1992). J. Chem. Phys. 96, 6010.
M. I. McCarthy, A. C. Hess, N. M. Harrison, and V. R. Saunders (1993). J. Chem. Phys. 98, 6387.
J.-Q. Lia, Y.-J. Xua, and Y.-F. Zhang (2003). Solid State Commun. 126, 107.
V. Ruangpornvisuti (2010). J. Mol. Model. 16, 1127.
J. Beheshtian, M. T. Baei, and A. A. Peyghan (2012). Surf Sci. 606, 981.
M. Schmidt, et al. (1993). J. Comput. Chem. 14, 1347.
C. Kim, B. Kim, S. M. Lee, C. Jo, and Y. H. Lee (2002). Phys. Rev. B. 65, 165418.
S. Li Semiconductor Physical Electronics, 2nd ed (Springer, Berlin, 2006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baei, M.T., Hashemian, S. Magnesium Oxide Nanotube as Potential Sensor for Cl2 Detection. J Clust Sci 24, 915–926 (2013). https://doi.org/10.1007/s10876-013-0590-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-013-0590-3