Abstract
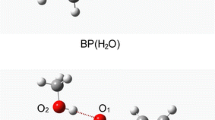
In this work, time-dependent density functional theory method was used to study the electronic transitions of hydrogen-bonded ethanol–water complexes Dimer-I, Dimer-II and Trimer. The intermolecular hydrogen bonds H1···O1 and O···H2 were demonstrated by the optimized geometric structures of the three hydrogen-bonded ethanol–water complexes. It is demonstrated that the S1-state electronic transitions for ethanol monomer and the hydrogen-bonded complex Dimer-I (through HB-I) should be of LE nature on the ethanol molecule, while those of complexes Dimer-II and Trimer should be of CT character from the hydrogen-bonded water molecule (through HB-II) to the ethanol moiety. The different electronic transition types should be the reasons for the tiny redshift of the S1-state electronic energy for Dimer-I and the large blueshifts for Dimer-II and the Trimer compared with that of the ethanol monomer.



Similar content being viewed by others
References
A. D. Buckingham, J. E. Del Bene, and S. A. C. McDowell (2008). Chem. Phys. Lett. 463, 1.
J. E. Del Bene (1978). J. Am. Chem. Soc. 100, 1395.
J. E. Del Bene (1980). Chem. Phys. 50, 1.
J. E. Del Bene (1981). J. Comput. Chem. 2, 422.
J. E. Del Bene (1981). J. Comput. Chem. 2, 188.
J. E. Del Bene (1982). J. Phys. Chem. 86, 1341.
J. E. Del Bene (1983). J. Comput. Chem. 4, 226.
J. E. Del Bene (1984). J. Mol. Strut. 108, 179.
J. E. Del Bene (1988). J. Phys. Chem. 92, 2874.
W. B. Person, J. E. Del Bene, W. Szajda, K. Szczepaniak, and M. Szczesniak (1991). J. Phys. Chem. 95, 2770.
J. E. Del Bene (1994). J. Phys. Chem. 98, 5902.
J. E. Del Bene, W. B. Person, and K. Szczepaniak (1995). J. Phys. Chem. 99, 10705.
J. E. Del Bene and M. J. T. Jordan (2001). J. Mol. Struc. 573, 11.
M. K. Ahmed, S. Ali, and E. Wojcik (2012). Spectrosc. Lett. 45, 420.
S. M. Mejia, E. Florez, and F. Mondragon (2012). J. Chem. Phys. 136, 144306.
J. R. Reimers and Z. L. Cai (2012). Phys. Chem. Chem. Phys. 14, 8791.
S. Alavi, S. Takeya, R. Ohmura, T. K. Woo, and J. A. Ripmeester (2010). J. Chem. Phys. 133, 074505.
S. Alavi, R. Ohmura, and J. A. Ripmeester (2011). J. Chem. Phys. 134, 054702.
S. Burikov, T. Dolenko, S. Patsaeva, Y. Starokurov, and V. Yuzhakov (2010). Mol. Phys. 108, 2427.
G. J. Zhao and K. L. Han (2007). J. Phys. Chem. A 111, 2469.
G. J. Zhao, J. Y. Liu, L. C. Zhou, and K. L. Han (2007). J. Phys. Chem. B 111, 8940.
G. J. Zhao and K. L. Han (2007). J. Phys. Chem. A 111, 9218.
G. J. Zhao and K. L. Han (2008). ChemPhysChem 9, 1842.
K.-L. Han and G.-J. Zhao, Hydrogen Bonding and Transfer in the Excited State (Wiley, Chichester, UK, 2010, ISBN: 978-0-470-66677-7), pp. 1–1072.
G. J. Zhao and K. L. Han (2008). Biophys. J. 94, 38.
Y.-F. Liu, D.-P. Yang, D.-H. Shi, and J.-F. Sun (2011). J. Comput. Chem. 32, 3475.
G. J. Zhao and K. L. Han (2009). J. Phys. Chem. A 113, 14329.
M.-X. Ma and D.-P. Yang (2012). J. Cluster Sci. 23, 155.
G.-J. Zhao and K.-L. Han (2012). Acc. Chem. Res. 45, 404.
B. M. Wong and T. H. Hsieh (2010). J. Chem. Theory Comput. 6, 3704.
M. J. G. Peach, E. I. Tellgren, P. Sałek, T. Helgaker, and D. J. Tozer (2007). J. Phys. Chem. A 111, 11930.
M. P. Balanay and D. H. Kim (2011). J. Phys. Chem. C 115, 19424.
B. M. Wong, M. Piacenza, and F. D. Sala (2009). Phys. Chem. Chem. Phys. 11, 4498.
W.-W. Zhao, Y.-H. Ding, and Q.-Y. Xia (2011). J. Comput. Chem. 32, 545.
J. Tomasi, B. Mennucci, and R. Cammi (2005). Chem. Rev. 105, 2999.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowskiand, and D. J. Fox Gaussian 09, Revision A02 (Gaussian Inc., Wallingford, CT, 2009).
P. A. Hunt, B. Kirchner, and T. Welton (2006). Chem. Eur. J. 12, 6762.
Acknowledgments
This work was supported by the Innovation Scientists and Technicians Troop Construction Projects of Henan Province of China; Grant No. 124200510013.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, D., Wang, H. Effects of Hydrogen Bonding on the Transition Properties of Ethanol–Water Clusters: A TD-DFT Study. J Clust Sci 24, 485–495 (2013). https://doi.org/10.1007/s10876-012-0514-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-012-0514-7