Abstract
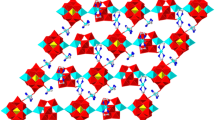
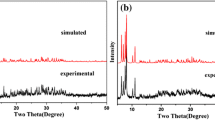
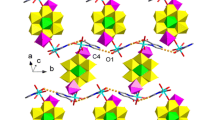
Two new Dawson-Type POTs, (H2en)H2[Cu(en)2][Cu(en)(H2O)3][Cu(en)2 (H2O)](P2W17CuO61)·8H2O (1, en = ethylenediamine) and H10K6(P2W17CuO61)2·34H2O (2), have been synthesized under hydrothermal conditions and characterized by IR spectroscopy, TG, XRD and single-crystal X-ray structural analysis. Crystal data for 1: triclinic, \( {P}{\bar{1}} \), a = 11.6855(26) Å, b = 13.2679(29) Å, c = 28.1540(60) Å, α = 80.4009(75)°, β = 82.5150(79)°, γ = 67.1482(46)°, Z = 2. Crystal data for 2: monoclinic, C2/c, a = 43.5701(99) Å, b = 12.3078(25) Å, c = 29.3742(66) Å, β = 101.3749(21)°, Z = 4. Compound 1 exhibits a 1D chain, in which the [Cu(en)2]2+ cations act as the linkages of mono-{P2W17Cu} units. Compound 2 shows a 2D layer, in which the double-Dawson units are connected by K+ cations.
Graphical Abstract
Two novel {P2W18} type Wells–Dawson-Type polyoxotungstate (H2en)H2[Cu(en)2][Cu(en)(H2O)3] [Cu(en)2(H2O)](P2W17CuO61)·8H2O (1) and H10K6(P2W17CuO61)2·34H2O (2) have been synthesized under hydrothermal conditions and solvent conditions. Compound 1 exhibit a 1D strait chain and Compound 2 exhibits a 2D layer structure based on double [P2W17CuO61]7− polyoxoanion linked by K+ cations.







Similar content being viewed by others
References
M. T. Pope Heteropoly and Isopoly Oxometalates (Springer, Berlin, 1983).
C.-L. Hill (Guest ed.) (1998). Special issue on Polyoxometalates. Chem. Rev. 98, 1–390.
M. T. Pope and A. Müller (eds.) Polyoxometalate Chemistry from Topology via Self-Assembly to Applications (Kluwer Academic Publishers, Dordrecht, 2001).
S. Landsmann, C. Lizandara-Pueyo, and S. Polarz (2010). J. Am. Chem. Soc. 132, 5315.
C.-Y. Duan, M.-L. Wei, D. Guo, C. He, and Q.-J. Meng (2010). J. Am. Chem. Soc. 132, 3321.
L.-C. Zhang, S.-L. Zheng, H. Xue, Z.-M. Zhu, W.-S. You, Y.-G. Li, and E.-B. Wang (2010). Dalton Trans. 39, 3369.
A. Harriman, K. J. Elliott, M. A. H. Alamiry, L. L. Pleux, M. Se′verac, Y. Pellegrin, E. Blart, C. Fosse, C. Cannizzo, C. R. Mayer, and F. Odobel (2009). J. Phys. Chem. C 113, 5834.
C. Jahier, S. Sankar Mal, U. Kortz, and S. Nlate (2010). Eur. J. Inorg. Chem. 10, 1559.
S. Reinoso, M. H. Dickman, A. Praetorius, L. F. Piedra-Garza, and U. Kortz (2008). Inorg. Chem. 47, 8798.
S.-T. Zheng, J. Zhang, and G.-Y. Yang (2008). Angew. Chem. Int. Ed. 47, 3909.
S.-T. Zheng, J. Zhang, J. M. Clemente-Juan, D.-Q. Yuan, and G.-Y. Yang (2009). Angew. Chem. Int. Ed. 48, 7176.
J.-W. Zhao, J. Zhang, S.-T. Zheng and G.-Y. Yang (2008). Chem. Commun. 44, 570.
B. Li, J.-W. Zhao, S.-T. Zheng, and G.-Y. Yang (2009). Inorg. Chem. 48, 8294.
R. Contant (1990). Inorg. Synth. 27, 108.
G. M. Sheldrick SHELXTL 97, Program for Crystal Structure Refinements (University of Göttingen, Germany, 1997).
G. M. Sheldrick SHELXS97, Program for Crystal Structure Solution (University of Göttingen, Germany, 1997).
G. M. Sheldrick SADABS Program for Siemens Area Detector Absorption Corrections (University of Göttingen, Germany, 1997).
Z.-M. Zhang, Y.-G. Li, Y.-H. Wang, Y.-F. Qi, and E.-B. Wang (2008). Inorg. Chem. 47, 7615.
J.-W. Zhao, B. Li, S.-T. Zheng, and G.-Y. Yang (2007). Cryst. Growth Des. 7, 2658.
B.-Z. Lin, L.-W. He, B.-H. Xu, X.-L. Li, Z. Li, and P.-D. Liu (2009). Cryst. Growth Des. 9, 273.
Z.-M. Zhang, S. Yao, Y.-F. Qi, Y.-G. Li, Y.-H. Wang, and E.-B. Wang (2008). Dalton Trans. 23, 3051.
Acknowledgments
The authors are thankful for the financial supports from the National Natural Science Fund for Distinguished Young Scholars of China (no. 20725101), the NNSF of China (no. 50872133), the NSF of Fujian Province (nos. E0510030) and the Knowledge Innovation Program from CAS (no. KJCX2.YW.H01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, L., Fang, WH., Li, XX. et al. Two New Dawson-Type Polyoxometalates: 1D Chain Made by Mono-Dawson Units and 2D Layer Made by Double-Dawson Units. J Clust Sci 22, 141–148 (2011). https://doi.org/10.1007/s10876-011-0366-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-011-0366-6